Insia Kizilbash1, Zonghu Han2, Srivasupradha Ramesh2, Bat-Erdene Namsrai3, Michael L. Etheridge2, Erik B. Finger3, John C. Bischof*2,4,5
1Department of Chemistry, University of Minnesota; Minneapolis, MN, USA
2Department of Mechanical Engineering, University of Minnesota; Minneapolis, MN, USA
3Department of Surgery, University of Minnesota; Minneapolis, MN, USA
4Department of Biomedical Engineering, University of Minnesota; Minneapolis, MN, USA
5Institute for Engineering in Medicine, University of Minnesota; Minneapolis, MN, USA
Abstract
Vitrification is a cryopreservation method that employs high concentrations of cryoprotective agents (CPA) to preserve organ viability indefinitely. Achieving vitrification typically requires CPA concentrations of 8–9M to effectively permeate tissues or organs; however, these levels are associated with significant toxicity. The CPA toxicity rate (k) quantifies the rate at which CPAs induce cytotoxic effects, influenced by CPA formulation, concentration and temperature. CPAs such as VM3 (8.46M), M22-PVP (9.34M) and M22 (9.35M) are currently under investigation for large organ cryopreservation. VM3 has been applied in kidney slice vitrification, while M22-PVP and M22 have been applied in rabbit kidney vitrification. This study evaluates the toxicity of these scalable CPAs at 4°C using rat kidney tissue slices. The slices were progressively exposed to increasing concentrations of VMP (a transitional CPA) to reach the final concentration, followed by exposure to VM3, M22-PVP or M22 for varying durations. Changes in tissue viability were measured, and toxicity rates were subsequently determined. VM3 exhibited the lowest toxicity rate (k = 0.007958min-1) compared to M22-PVP (k = 0.01755min-1) and M22 (k = 0.02339min-1). The findings from this study, combined with future research on the temperature dependence of CPA toxicity, will drive the development of long-term human kidney banking for transplantation,enhancing donor-recipient matching, promoting equitable access, refining patient preparation and tolerance induction protocols, optimizing organ utilization and ultimately improving survival outcomes.
Introduction
Ischemic damage affects all transplantable organs, posing a significant barrier to addressing the organ shortage crisis. Damage to the organ begins as soon as the organ is harvested from the donor; hence, quick efforts to maintain viability are the foremost priority for successful transplantation. Currently, ischemic time limits are short: up to 4 hours for hearts, 8–12 hours for livers and 36 hours for kidneys (Finger & Bischof, 2018). These limited windows create logistical challenges, often resulting in organ wastage, financial losses and threats to patient survival. Long-term organ banking, particularly through cryopreservation, offers a promising solution. However, the biggest threat to using cryopreservation is the formation of ice crystals that can damage structures within tissues and cells (Finger and Bischof 2018). It has been observed that organisms are able to sustain life below freezing temperatures. In nature, we observe organisms ranging from microscopic invertebrates to cold-adapted species, such as polar fish, that have been able to sustain life in such adverse conditions (Devries 1971). One attribute common amongst these organisms is the ability to prevent ice formation to sustain life. Two different mechanisms to avoid ice are ice tolerance and ice avoidance. Ice tolerance allows the formation of ice, however it directs the ice formation to other parts of the body in a non-destructive manner (Plitz, Rabin et al. 2004). Ice avoidance focuses on the inhibition of ice. Cells produce antifreeze proteins to lower the freezing temperature and inhibit the propagation of intracellular ice (Mehl 1993). This, in turn, also prevents recrystallization. This mechanism is seen most often in polar fish (Somero and DeVries 1967, Knight, De Vries et al. 1984).
Researchers have applied these mechanisms to cryopreservation. Two different techniques are used within cryopreservation: 1) conventional cryopreservation and 2) vitrification. Conventional cryopreservation allows the formation of ice crystals; however, ice is controlled and preferentially formed in the extracellular spaces much like ice tolerance (Mazur 1963, Benson, Kearsley et al. 2012). Vitrification, an ice-free cryopreservation approach, involves the avoidance of freezing at vitreous temperatures (e.g., -150°C) theoretically to indefinitely extend the viability of organs through cryopreserved storage (Mazur 1984, Probst, Becerra et al. 2015, Finger and Bischof 2018). Phase transition occurs when a substance transitions from solid to liquid upon heating and, similarly, when a liquid is cooled to form a solid or crystalline state like ice (Han, Gangwar et al. 2022). Vitrification applies rapid cooling, and thus liquid can enter a stable, non-crystalline glass-like state instead of a crystalline solid state. The transition temperature at which this transition takes place is called glass transition temperature (Tg) (Mazur 1984, Probst, Becerra et al. 2015, Finger and Bischof 2018).
Rapid cooling mitigates ice formation. Decreasing the temperature increases the solution viscosity and decreases the ability for ice nucleation and ice growth. CPAs are ice inhibitors. The critical cooling rate (CCR) is the temperature change rate required between the homogeneous nucleation temperature (Th) and glass transition temperature (Tg) to achieve vitrification. Similarly, critical warming rate (CWR) is the temperature change rate required between Tg and Th during rewarming to avoid ice formation. The homogeneous nucleation temperature refers to the transition temperature at which ice nuclei form spontaneously. The CCR of water is extremely high, making the water vitrification impractical to achieve; as a result, CPAs are used to lower the CCR, thus making vitrification achievable (Mazur 1984, Probst, Becerra et al. 2015, Finger and Bischof 2018).
Despite their important ability to facilitate vitrification, CPAs can cause toxicity in biological systems and damage due to osmotic cell volume changes (Fahy, Wowk et al. 2004). A toxicity cost function was introduced to explore the cumulative toxicity in cells and tissues. The degree to which CPA addition and removal steps induce toxicity depends on CPA formulation, duration of exposure, the CPA concentration and temperature. A minimally toxic cost function requires taking exposure time and CPA concentrations into consideration (Benson, Kearsley et al. 2012, Davidson, Glasscock et al. 2015). This study investigates the relative toxicity of various CPAs on kidney tissue, hypothesizing that agents with lower toxicity cost functions will result in higher tissue viability post-vitrification.
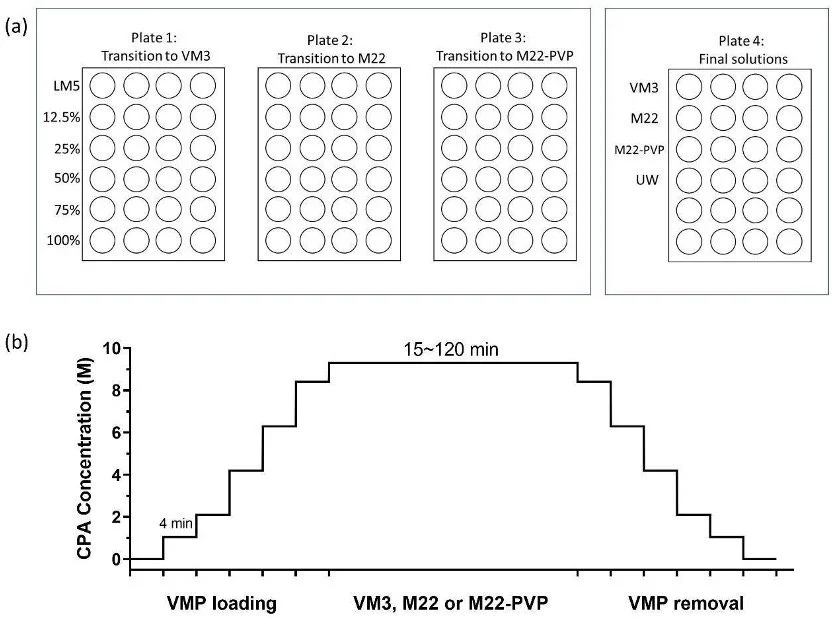
VM3 is a general-purpose CPA that has been successfully applied to kidney slices vitrification, where the vitrified kidney slices were able to maintain viability at 50–80% of fresh slice levels (de Graaf, Draaisma et al. 2007). In comparison, M22 is designed for kidneys and has been successfully applied to whole rabbit kidney vitrification achieving successful transplantation of rabbit kidneys after vitrification and rewarming (Fahy, Wowk et al. 2009, Wowk, Phan et al. 2024). Subsequently, M22-PVP (meaning M22 minus PVP) was developed to further reduce toxicity (Fahy, Wowk et al. 2004). All these three CPAs are vitrifiable on the human-organ scale. Alongside the three scalable CPAs (VM3, M22-PVP, M22), VMP is included as a comparative control, as VMPhas already demonstrated acceptable toxicity for transplant in vitrified and rewarmed rat kidneys (Han, Rao et al. 2023). In addition, it is used as a precursor in loading the higher molarity CPAs, since it has similar components but is less concentrated. The tissues are gradually exposed to increasing concentrations of VMP and then exposed to the higher concentration CPA in the final step, as shown in Figure 1b.
As mentioned before, although CPAs can cause damage to the organ, they are essential to perform vitrification successfully. To mitigate the damage caused by CPAs, knowing which CPA is least damaging to the organ is essential. There are a few standard equations used to derive the toxicity cost function (Benson, Kearsley et al. 2012).
$$I_{\text{tox}} = \int_0^{t_f} k \, dt = \int_0^{t_f} \beta C^{\alpha} \, dt$$ (1)
$$k = \beta C^{\alpha}$$ (2)
$$\frac{dN}{dt} = -kN$$ (3)
In Equation (1), Jtox represents the toxicity cost function, with lower values indicating a more optimal CPA for use. The variable tf is the duration of CPA exposure. The k value is the toxicity rate and is dependent on CPA formulation, concentration and temperature. The toxicity rate and toxicity function itself are directly dependent. As a result, a lower toxicity constant indicates lowered toxicity. The parameters β and α depend on the type of CPA used. The C value represents the CPA concentration that is inside the tissue. Equation (2) is an equation for toxicity rate and is substituted into the toxicity cost function. Equation (3) models cell viability as a function of CPA exposure, where N represents cell viability after exposure. Combining Equations (2) and (3), the following equation can be derived:
$$\frac{N}{N_0} = \exp(-I_{\text{tox}})$$ (4)
Equation (4) is a representation of cell viability from the beginning of exposure time N0 to the end of exposure time, N, representing a decreasing exponential function displayed by the negative Jtox value. We hypothesize that the use of CPAs with lower toxicity cost functions will result in higher cell viability post-vitrification compared to CPAs with higher toxicity cost functions. Moreover, the CPAs with the least cumulative toxicity, as indicated by their toxicity cost function values, will result in less damage to the tissue, thereby improving the overall success of long-term organ banking through vitrification. By testing this hypothesis, we aim to identify the most effective CPA for long-term organ preservation, optimizing both the vitrification process and the viability of organs for transplantation.
Method
Preparation of tissues
We procured the kidneys from 3-month-old Sprague-Dawley rats, consistent with those used in previous studies that achieved success in rat kidney transplantation (Han, Rao et al. 2023). All procedural protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota. The kidneys were rinsed briefly with 0.9% normal saline solution to remove residual blood and placed in cold UW solution for transportation. Kidney tissue slices were obtained using a tissue slicer at a thickness of 300µm, a thickness that allows sufficient entry of nutrients and oxygen, preventing necrosis of the inner cell layers (Graaf, Groothuis et al. 2007). Sixteen tissue slices were needed for each experiment and can be stored in UW solution on ice. Each slice was put into a well with 500µL of DMEM (Dulbecco's Modified Eagle Medium, culture solution) and incubated for an hour to rest at 37°C and 5% CO2/95% air. Tissues were then transferred to wells that were filled with mixed solution of 450µL of DMEM and 50µL of non-destructive alamarBlue assay to measure baseline mitochondrial activity. An extra well was needed this time for the background value. Tissues are then incubated for 50 minutes. The fluorescence of each well was measured using a plate reader (Synergy HT, BioTek) with excitation/emission wavelengths of 530/590nm.
CPA preparation
All the carrier and CPA solutions were prepared in the lab. LM5-XZ [LM5 + 1% X-1000 (w/v) and 1% Z-1000 (w/v)] was used as the carrier solution to dilute VMP. The polymers X-1000 and Z-1000, needed for preparing VMP and LM5-XZ, were purchased (21st Century Medicine, Fontana, CA). VMP (8.44 M), VM3 (8.46 M), M22-PVP (9.34 M) and M22 (9.35 M) were used in this study, where VMP was only used for step loading and removal as the transitional CPA to reduce the osmotic damage. VMP contains 16.84% (w/v) ethylene glycol, 12.86% (w/v) formamide, 22.3% (w/v) DMSO (dimethyl sulfoxide), 1% (w/v) X-1000 and 1% (w/v) Z-1000. VM3 contains an additional 7% PVP K12 compared to VMP. M22 contains 16.84% (w/v) ethylene glycol, 12.86% (w/v) formamide, 22.3% (w/v) DMSO, 3% (w/v) N-Methylformamide, 4% (w/v) 3-Methoxy,1,2-propanediol, 2.8% (w/v) PVP K12, 1% (w/v) X-1000 and 2% (w/v) Z-1000. M22-PVP contains every component in M22 except for PVP K12.
CPA addition and removal
Four different 24-well plates were used as part of the step-loading process toward the exposure of the scalable CPAs. Plates 1, 2 and 3 each contained the same step-loading solutions (1mL in each well), and tissues from each of these plates ended up resting in one of the three final testing CPAs, as shown in Figure 1a. For example, tissues from Plate 1 transition to VM3, Plate 2 transition to M22 and so on. The step-loading solution was VMP, and it increased in concentration with each column. The solutions were kept on ice, maintaining a loading temperature of 0–4°C, as measured by a thermocouple throughout the entire experiment.
Figure 1. The toxicity measurements of VM3, M22 and M22-PVP: (a) This is the representation of 24 wells used for CPA loading and unloading procedures. We kept the tissues in one row of wells in LM5 and then moved them to the next row of wells simultaneously going to higher concentration. (b) CPA loading and removal protocol, where VMP loading and removal steps are added before and after the testing solution (VM3, M22 or M22-PVP) step to avoid osmotic damage. The number represents the time spent in each well with increasing concentrations.
Tissues from Plate 1, 2 and 3 rest within the same row simultaneously for 4 mins each based on the equation tchar = l2/(2D), where l is the characteristic length of the slice (half-thickness = 150μm), and D is the diffusivity, 6.5 × 10-11 m2/s (Han, Sharma et al. 2020). We exposed the tissues to the final CPA solution for a specified amount of time (15, 30, 45, 60, 90 or 120 mins). Then, we transferred them back to 100% VMP and started the step removal process going backward to increasingly diluted solutions. The control group was rested within the UW solution for the whole experiment. Upon completion of the removal procedure tissues were again put in 500 µL of DMEM and incubated for 50 minutes followed by DMEM and alamarBlue for 50 minutes again and then fluorescence was measured. Data gathered through the plate reader were compared to the original reading without treatment to represent the tissue viability. These values were then further normalized to UW controls to quantify the toxicity introduced by the CPA.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism (GraphPad Software, Inc.). The number of biological replicates is specified in each figure legend, with all measurements representing distinct biological replicates obtained from individual kidneys. Toxicity rates were calculated by fitting the viability data to exponential decay models using GraphPad Prism.
Results
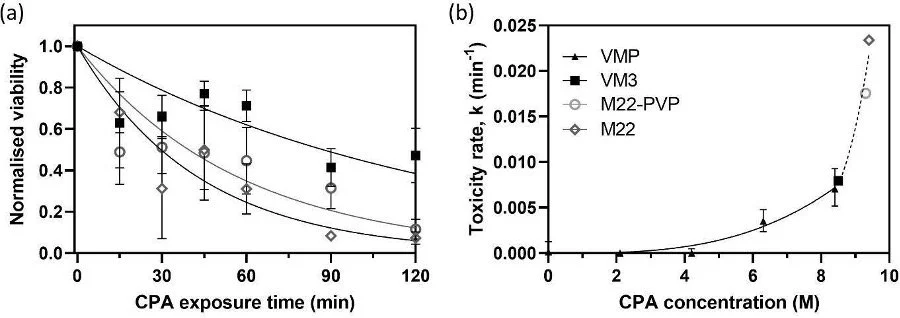
For the three CPAs measured in this study, the viability reduction of kidney slices over time can be observed clearly, as shown in Figure 2a. VM3 consistently demonstrates the highest viability, represented by the top line, indicating the lowest observed toxicity. In contrast, M22 shows the lowest viability, represented by the bottom line, highlighting its relatively high toxicity under the tested exposure conditions. For each CPA, viability data were fitted to an exponential decay model, as described by Equation (4) in the introduction, to determine the corresponding toxicity rates. These toxicity rates were then plotted against CPA concentration in Figure 2b.
Figure 2. Toxicity cost function measurements. (a) Tissue viability was measured across different exposure times for VM3, M22 and M22-PVP, with n = 4 for each concentration at each exposure time. The lines represent the best-fit curves according to Equation (3). (b) Toxicity rates are plotted as a function of CPA concentration, showing a positive correlation between increasing concentration and toxicity rate.
The toxicity rate values for VMP have been reported before and replotted here for comparison. The new toxicity rates (k) obtained from this paper for VM3, M22-PVP and M22 are 0.007958, 0.01755 and 0.02339min-1, respectively. Compared to the previously obtained toxicity rate for VMP (0.007051min-1) (Han, Rao et al. 2023), all three CPAs presented more toxicity to the kidneys due to the additional permeating and/or non-permeating CPA components. In addition, the solid fitting curve representing VMP toxicity rate dependence on concentrations and the dashed fitting curve for the three scalable CPAs follow a similar trend in general, which agrees with previous observations (Benson, Kearsley et al. 2012, Davidson, Glasscock et al. 2015).
Discussion
Recently, Han et al. (2023) achieved the first case of successful vitrification and rewarming of rat kidneys, followed by reproducibly survivable transplantations using the CPA, VMP (Han, Rao et al. 2023). However, VMP is insufficient for vitrifying human-sized kidneys without ice formation. Scaling up to human-sized organs necessitates identifying a more concentrated CPA capable of vitrification at the cooling and rewarming rates feasible for larger kidneys while maintaining acceptable toxicity levels. In this study, we tested the toxicity rate of three scalable CPA candidates, VM3, M22-PVP and M22, to potentially guide the development of vitrification and rewarming protocols for human-sized kidneys.
Human-sized kidneys can be cooled at ~2–4°C/min (depending on their sizes) in the core region, therefore a CPA with CCR < 2°C/min can be generally defined as a scalable CPA (Fahy and Wowk 2014). In general, CWR is multiple-fold (~ 5-fold in the concentration of these scalable CPAs) higher than the CCR, e.g., M22 was reported to have a CCR of 0.1°C/min and CWR of 0.4°C/min (Fahy, Wowk et al. 2006). The CWR of VM3 was reported to be 3.8°C/min (Fahy, Wowk et al. 2004); therefore the CCR can be estimated to be < 1°C/min. The CCR and CWR for M22-PVP have not been reported, but can be estimated to be < 0.5°C/min and ~1°C/min, respectively, based on its concentration (Han and Bischof 2020). Therefore, all these three CPA candidates are scalable CPAs for human-sized kidneys.
Exposure to high CPA concentrations causes injury to tissues by two mechanisms: mechanical (osmotic) damage and chemical (toxicity) damage. Osmotic damage can be reduced by controlling the rates at which CPA is added and removed, to prevent excessive volume excursions caused by transient intra- versus extracellular concentration differences. In this study, VMP is used as the transitional CPA in stepping to the higher CPA concentrations to eliminate osmotic damage. Therefore, the change in mitochondrial activity before and after the addition and removal of CPA measured by alamarBlue solely reflects the chemical toxicity originating from the exposure to these high-concentration CPAs.
Figure 2 displays the toxicity rate for VMP, VM3, M22-PVP and M22. There is a clear positive correlation between CPA toxicity and CPA concentration. Moreover, the new toxicity data for the three concentrated CPAs (VM3, M22-PVP and M22) follow the published VMP trend, which is interesting. This could be because they are all composed of similar components. This suggests that one may be able to make a first-order prediction of toxicity for an M22-derivative CPA’s toxicity rate within this concentration range (0–9.35M), which could be very beneficial for future CPA design. Using the previously published toxicity-concentration relationship of VMP (0–8.44M), Han et al. (2023) were able to optimize the VMP loading protocol by shortening the perfusion duration and reducing the toxicity, and achieved successful transplantations (Han, Rao et al. 2023). With the expansion of the toxicity-concentration curve to a wider range (0–9.35M), we will be able to design an optimal loading protocol for the scalable CPAs.
VM3 was identified as the least toxic CPA among those tested and will be evaluated further for vitrification of larger organs. However, identifying the least toxic CPA is only one aspect of minimizing overall toxicity. Other factors, such as temperature, can also be explored to enhance organ viability. All the CPA toxicities were only tested at 4°C in this study, however, not all of the CPAs were designed at 4°C, e.g., M22 was designed for -22°C (Fahy, Wowk et al. 2009). Reducing temperature can significantly decrease the toxicity (Davidson, Glasscock et al. 2015), therefore, exploring the temperature-dependence of CPA toxicity (e.g., for VM3) can be very beneficial to building a multi-thermic perfusion system where temperature adjustments at various CPA concentrations are used to achieve minimal toxicity. This approach will facilitate the development of vitrification and nanowarming protocols for human-sized kidneys, ultimately advancing the goal of long-term organ banking.
The main limitation of this study is that CPA toxicity was assessed using a single in vitro approach, alamarBlue, which may not fully represent in vivo toxicity during kidney perfusion. Previous studies by Fahy et al. (2004) have evaluated the toxicity of VM3 and M22 in kidney slices using the K+/Na+ ratio, a marker primarily indicative of membrane integrity (Fahy, Wowk et al. 2004). Additionally, VMP and M22 have been perfused into whole kidneys to assess functionality (Fahy, Wowk et al. 2004). In this study, mitochondrial activity was employed as a viability marker to measure the toxicity of the three CPAs, and the observed relative toxicity trends were consistent with previous findings using K+/Na+ ratio and kidney functionality assessments, thereby validating our results. Nonetheless, further studies employing diverse approaches are necessary to comprehensively evaluate CPA toxicity.
Conclusions
Ischemic injury remains a critical challenge in transplantation medicine, and effective cryopreservation strategies offer promising prospects for long-term organ storage. A significant barrier to the advancement of cryopreservation is ice formation, which can be mitigated through vitrification. In this study, we evaluated and compared the toxicity profiles of several CPAs considered for organ vitrification protocols. Our findings demonstrate that VM3 exhibits the lowest toxicity among the CPAs tested under the specified exposure conditions. Notably, CPA toxicity and permeability are temperature-dependent, suggesting that future investigations should focus on the impact of temperature. Additionally, exploring CPA loading dynamics based on mass transport properties within tissues may further refine vitrification protocols.
Acknowledgments
Thank you Zonghu Han for providing your incredible mentorship throughout this project. This work was supported by NSF grant EEC-1941543, NIH grants DK117425 and DK132211.
References
Benson, J. D., A. J. Kearsley and A. Z. Higgins (2012). "Mathematical optimization of procedures for cryoprotectant equilibration using a toxicity cost function." Cryobiology 64(3): 144-151.
Davidson, A. F., C. Glasscock, D. R. McClanahan, J. D. Benson and A. Z. Higgins (2015). "Toxicity minimized cryoprotectant addition and removal procedures for adherent endothelial cells." PloS one 10(11): e0142828.
de Graaf, I. A., A. L. Draaisma, O. Schoeman, G. M. Fahy, G. M. Groothuis and H. J. Koster (2007). "Cryopreservation of rat precision-cut liver and kidney slices by rapid freezing and vitrification." Cryobiology54(1): 1-12.
Devries, A. L. (1971). "Glycoproteins as biological antifreeze agents in Antarctic fishes." Science 172(3988): 1152-1155.
Fahy, G. M. and B. Wowk (2014). Methods and compositions for the cryopreservation of organs, Google Patents.
Fahy, G. M., B. Wowk, R. Pagotan, A. Chang, J. Phan, B. Thomson and L. Phan (2009). "Physical and biological aspects of renal vitrification." Organogenesis 5(3): 167-175.
Fahy, G. M., B. Wowk and J. Wu (2006). "Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain." Rejuvenation research 9(2): 279-291.
Fahy, G. M., B. Wowk, J. Wu and S. Paynter (2004). "Improved vitrification solutions based on the predictability of vitrification solution toxicity." Cryobiology 48(1): 22-35.
Fahy, G. M., B. Wowk, J. Wu, J. Phan, C. Rasch, A. Chang and E. Zendejas (2004). "Cryopreservation of organs by vitrification: perspectives and recent advances." Cryobiology 48(2): 157-178.
Finger, E. B. and J. C. Bischof (2018). "Cryopreservation by vitrification: a promising approach for transplant organ banking." Current Opinion in Organ Transplantation 23(3): 353-360.
Graaf, I. A. d., G. M. Groothuis and P. Olinga (2007). "Precision-cut tissue slices as a tool to predict metabolism of novel drugs." Expert opinion on drug metabolism & toxicology 3(6): 879-898.
Han, Z. and J. C. Bischof (2020). "Critical cooling and warming rates as a function of CPA concentration." CryoLetters 41(4): 185-193.
Han, Z., L. Gangwar, E. Magnuson, M. L. Etheridge, C. O. Pringle, J. C. Bischof and J. Choi (2022). "Supplemented phase diagrams for vitrification CPA cocktails: DP6, VS55 and M22." Cryobiology 106: 113-121.
Han, Z., J. S. Rao, L. Gangwar, B.-E. Namsrai, J. L. Pasek-Allen, M. L. Etheridge, S. M. Wolf, T. L. Pruett, J. C. Bischof and E. B. Finger (2023). "Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model." Nature Communications 14(1): 3407.
Han, Z., J. S. Rao, S. Ramesh, J. Hergesell, B.-E. Namsrai, M. L. Etheridge, E. B. Finger and J. C. Bischof (2023). "Model-guided design and optimization of CPA perfusion protocols for whole organ cryopreservation." Annals of Biomedical Engineering 51(10): 2216-2228.
Han, Z., A. Sharma, Z. Gao, T. W. Carlson, M. G. O'Sullivan, E. B. Finger and J. C. Bischof (2020). "Diffusion limited cryopreservation of tissue with radiofrequency heated metal forms." Advanced healthcare materials 9(19): 2000796.
Knight, C. A., A. L. De Vries and L. D. Oolman (1984). "Fish antifreeze protein and the freezing and recrystallization of ice." Nature 308(5956): 295-296.
Mazur, P. (1963). "Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing." The Journal of general physiology 47(2): 347-369.
Mazur, P. (1984). "Freezing of living cells: mechanisms and implications." American journal of physiology-cell physiology 247(3): C125-C142.
Mehl, P. M. (1993). "Nucleation and crystal growth in a vitrification solution tested for organ cryopreservation by vitrification." Cryobiology 30(5): 509-518.
Plitz, J., Y. Rabin and J. R. Walsh (2004). "The effect of thermal expansion of ingredients on the cocktails VS55 and DP6." Cell Preservation Technology 2(3): 215-226.
Probst, C. P., A. Z. Becerra, C. T. Aquina, M. A. Tejani, S. D. Wexner, J. Garcia-Aguilar, F. H. Remzi, D. W. Dietz, J. R. Monson and F. J. Fleming (2015). "Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation." Journal of the American College of Surgeons 221(2): 430-440.
Somero, G. N. and A. L. DeVries (1967). "Temperature tolerance of some Antarctic fishes." Science156(3772): 257-258.
Wowk, B., J. Phan, R. Pagotan, E. Galvez and G. M. Fahy (2024). "27 MHz constant field dielectric warming of kidneys cryopreserved by vitrification." Cryobiology 115: 104893.