Authors: Stephen McAuliffe, José Iván Martes Martinez, Laura Warman, and Rebecca Ostertag
Institution: University of Hawai'i Hilo
Erratum, Apr 2016: L. Warman and R. Ostertag added as coauthors.
ABSTRACT
Hawaiian lowland wet forests (HLWF) have been subject to degradation as a result of human activities and the introduction of non-native plant and animal species. Arthropods play crucial roles in forest ecological processes and food web dynamics, and this study aims to compare arthropod diversity and herbivory in HLWF with high or low degrees of invasion. The objective of this study is to test whether invaded HLWF have greater arthropod diversity and therefore higher rates of herbivory than HLWF undisturbed by invasion. In addition, it is hypothesized that invasive plants have higher levels of herbivory due to their pioneer traits, which involve fast growth and low defense physiology. We set up two types of arthropod traps, and herbivory and leaf traits of two native and two non-native plants were assessed at both forest sites. Contrary to our hypotheses, we observed a higher variety of arthropods and higher levels of herbivory at the primarily native site. Primarily native forests may provide more fitting environments for arthropods than invaded forests.
INTRODUCTION
Forest ecosystems support astoundingly rich arthropod abundances. Aside from direct nutritional value to herbivores, trees provide arthropods with shelter, as well as sites for foraging, oviposition, sun-basking, sexual display, and overwintering (Halaj, Ross, & Moldenke, 2000). In reciprocity, arthropods take part in a series of ecological processes such as leaf litter decomposition and competition for resources (Olofssn, Hulme, Oksanen, & Suominen, 2004). They also play vital roles in food web dynamics as herbivores, decomposers, predators, and prey. Arthropods are large contributors to herbivory in forests (Basset, 1991), and one of the most important selective forces in the evolution of plants is their susceptibility to herbivory (Coley, 1983).
Due to the geographic isolation and geologic origins of the Hawaiian Islands, their floral and faunal biodiversity are considered disharmonic. In other words, these islands evolved and continue to evolve separately from each other and may lack ecological symmetry. This has resulted in a collection of native flora with few fast-growing pioneer species and a low species richness level of native arthropods (Gillespie & Roderick, 2002). However, over the past one hundred years, the accidental and deliberate introduction of non-native plant and animal species has led to forest degradation and a decline of native species (Cordell et al., 2009). Introduced species now represent approximately 50% of the floral diversity on the Hawaiian archipelago (Wagner, Herbst, & Sohmer, 1999). Some introduced plants have become invasive and are able to outcompete natives because they use strategies to which local species cannot effectively respond.
Studies conducted in other tropical forests have shown that certain characteristics make leaves more palatable to herbivores. For example, young leaves are softer, have greater water content, and have greater nutritional value than mature leaves, and herbivory is demonstrably greater in younger leaves (Coley, 1983). Likewise, gap-colonizing or pioneer species, which tend to have softer leaves, are grazed six times more rapidly than shade-tolerant species (Coley, 1983). Many of the invasive species that now dominate Hawaiian lowland wet forests (HLWF) are considered pioneer species (Zimmerman et al., 2008), which means they may face higher herbivory rates than native species in Hawai’i. Because Hawai’i has no native mammalian herbivores and most of the grazing birds are now extinct (James & Burney, 1997), this study focuses on herbivory damage produced by arthropods.
Invasive species are well known for their disruptive biotic impact on native ecosystems. As invasive species increase in abundance, native plant diversity and density often measurably decline along with herbivore populations (Zagrobelny, Bak, & Moller, 2008). However, an introduction of species could also foster greater diversity in arthropods (Mascaro, Hughes, & Schnitzer, 2012). Invasive plants often have different chemical defenses than native plants, which can prevent or reduce herbivory by native fauna. This may cause a decline in the herbivore population or allow for adaptations in herbivores to occur (Zagrobelny et al., 2008). The introduction of non-native plants may also lead to an increase in leaf litter, which in turn may result in an increased population of ground-dwelling arthropods. In their research on springtail abundance in a forest invaded by garlic mustard, Alerding and Hunter (2013) found that springtail populations tripled in invaded areas compared to non-invaded regions due to increased leaf litter. Arthropod abundance contributes largely to forest herbivory and decomposition. However, little is known about what determines their abundance and herbivory habits regarding plant species (Halaj et al., 2000).
This study aims to compare arthropod diversity, measured by abundance and variety, and herbivory in mostly native and highly invaded HLWF. Two types of traps were set up to quantify arthropod populations, and herbivory was assessed on four plant species in each forest type. We hypothesized that invaded forest has a higher abundance and greater variety of arthropods than primarily native forest. Consequently, herbivory rates should be higher in the invaded forest than in the primarily native forest. In addition, we predicted that non-native plant species have higher herbivory rates than native plant species due to differences in leaf traits, including leaf size and water content.
MATERIALS & METHODS
Study Sites
Two sites on the eastern (windward) side of Hawai’i Island were included in this study. The invaded forest site is the lowland wet forest at the Keaukaha Military Reserve (KMR) located in the town of Hilo, just south of the Hilo Airport (19.704975 N, -155.039743 W). This forest is established atop a 750 to 1,500-year-old ‘a‘ā (“rough and blocky”) lava flow with an average rainfall of 3,280mm per year and canopy height ranging from 23 to 35m (Ostertag et al., 2009). The native trees ‘ōhi‘a (Metrosideros polymorpha) and lama (Diospyros sandwicensis) dominate the canopy, and various shrubs, trees, and ferns inhabit the mid- and understory. The forest has been invaded by numerous non-native species, including the trees strawberry guava (Psidium cattleianum), bingabing (Macaranga mappa), albizia (Falcataria moluccana), and melastoma (Melastoma septemnervium), as well as the shrub Koster’s curse (Clidemia hirta) (Ostertag et al., 2009).
The native forest site, Keau‘ohana State Forest Reserve, is located south of Pahoa in the Puna District of Hawai’i Island (19.415755 N, -154.952084 W). This study focused on the 200- to 400-year-old ‘a‘ā lava flow section of the reserve, which receives an average rainfall of 2,500mm per year (Hughes & Denslow, 2005). The canopy at this native site is also dominated by ‘ōhi‘a (M. polymorpha) and lama (D. sandwicensis), as well as kōpiko (Psychotria hawaiiensis), māmaki (Pipturus albidus), and various ferns, both native and non-native, in the mid- and understory. This forest has not experienced the same degree of disturbance or invasion as the invaded site, and its primarily native status has been maintained by periodic removals of invasive species by the local community.
Arthropod Sampling
At each site, two intersecting 30m transects were positioned to collect arthropods. The transects were arranged in north-south and east-west orientations so that the 15m marks of both transects overlapped. Colored sticky traps were placed at the apex of each transect in order to capture arthropods at the vegetative level. Each trap consisted of a 10cm by 10cm square of colored construction paper (orange, red, yellow, blue or green) marked with a 1cm by 1cm grid. The trap was wrapped in transparent plastic for weatherproofing and covered in Tree Tanglefoot (Contech, Victoria, Canada) to capture arthropods. At each sampling station, the five traps were hung at 20cm intervals along a 1m dowel, which were suspended 1m above the forest floor. The colored traps were arranged in random order along the dowel.
In addition to the sticky traps, pitfall traps were installed to trap ground-dwelling arthropods. Conical centrifuge tubes (50mL capacity) partially filled with soap and water served as pitfall traps. Five pitfall traps were placed 1m apart on a 5m ground transect at the apex of each 30m transect below the sticky fly traps. The fifth pitfall trap was positioned at the overlapping centers of the two 30m transects. The traps were collected after 7d and the arthropods were identified to their respective order using a dissecting microscope and identification key (Jaques, 1947). The data from both trap types were analyzed using one-way analysis of variance (ANOVA) in R (Version 2.15.0; R Core Team, 2013).
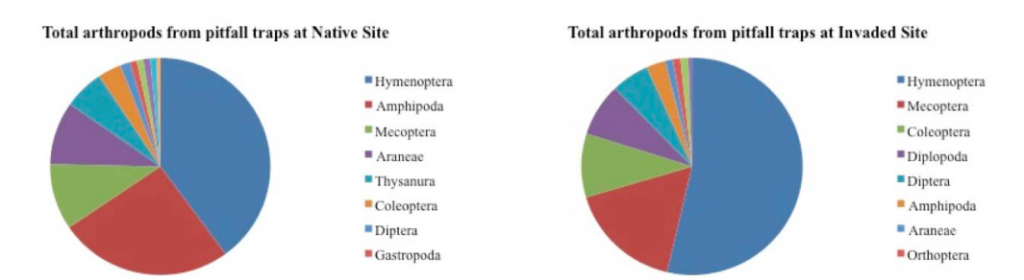
Figure 1. Abundance across sites was significant with almost twice as many arthropods found at the native site as at the invaded site (p = .010). Pitfall traps showed significant differences in variety, with more Araneae (p = .063) and Amphipods (p = .045) found in the native site, and Diplopoda (p = .003) found only in the invaded site.
Measures of herbivory
At both sites leaves were collected from native and non-native species in order to compare the amount of herbivory damage. Four plant species were selected based on native or non-native origin, similarity of life history and traits, and abundance. The native species were māmaki (P. albidus) and kōpiko (P. hawaiiensis) and the invasive species selected were strawberry guava (P. cattleianum) and Koster’s curse (C. hirta). No adult māmaki plants were present at the invaded site during sampling, so this species was only collected from the native site.
At least 150 leaves from each species were randomly collected at each site. A maximum of three leaves were chosen per individual plant. Edge areas of the forest were avoided during the collection, and only fully enlarged adult leaves from relatively sunny positions were collected.
In the lab, 100 leaves from each species were selected for analysis using a random number generator. Leaves were scanned using a flatbed scanner, and leaf area was calculated using ImageJ (Version 1.47t; U.S. National Institutes of Health, 2014), a digital image processing software. ImageJ was also used to quantify the existing amount of herbivory damage, or total area of holes within the leaf, by drawing the assumed outline of the leaf and calculating the missing area as herbivory. To obtain both wet and dry weights, the leaves were weighed using an electronic balance after scanning and after being dried at 70°C for at least 48hr. Leaf mass per unit area (LMA) was calculated as the ratio of dry weight to leaf area for each species.
Figure 2. Herbivory by species the native and invaded sites. No significance was found in the observed species save for C. herta, an invasive understory plant (t = 2.15, p = .034).
Statistical analysis
Herbivory and leaf trait values in both native and invasive species and herbivory across sites were compared using two-sample t-tests in Minitab® Statistical (Version 16; Minitab Inc., 2010). One-way ANOVA was used to compare values across species. The threshold for statistical significance was p < .05. P. albidus was excluded from comparisons across sites because it was only found at one site.
RESULTS
Abundance of Arthropods
The sticky fly traps showed significant differences in arthropod abundance between the two sites (F = 10.93; p = .01). No differences were found in arthropod color preferences for traps. Traps at the native site had nearly twice as many arthropods as traps at the invaded site (365 and 196, respectively). Total arthropod abundance from pitfall traps showed no statistical significance between sites (F = 0.365, p = .56), although there were differences in abundance of some orders between sites. Numbers of Amphipoda and Thysanura were almost an order of magnitude higher at the native site (n = 52 and n = 5, respectively; F = 5.642, p = .045) than at the invaded site (n = 12 and n = 2, respectively; F = 12.5, p = .008). In addition, Araneae and Mecoptera showed strong trends across sites with higher abundances at the native site (F = 0.62, p = .064, and F = 5.00, p = .056, respectively).
Diversity of Arthropods
There was no statistical significance in the variety of arthropod orders between sites in either of the trap types (F = 0.365, p = .563). However, Diplopoda was only observed in the invaded site, while Protura and Ephemeroptera were only observed in the native site (Figure 1). Of note, gastropods, although not arthropods, were also only observed in the native site.
Figure 3. Average herbivory damage in the native site compared to the invaded site. We observed significantly higher damage area in the native site (p = .036). This may be due to the damaged sustained by C. herta, which endured roughly six times more damage in the native site than in the invaded site.
Herbivory and Leaf Traits
Overall, there was no significant difference among native and invasive plant species in percent damage (Figure 2). There were strongly significant differences between the sites. Herbivory damage was nearly five times greater and significantly higher in the native forest than in the invaded forest (t = 2.11, p = .036; Figure 3). C. hirta leaves sustained roughly six times more damage in the native forest than in the invaded forest (t = 2.15, p = .034). Two other species, P. hawaiiensis and P. cattleianum, did not demonstrate significant differences in percent damage between forest types. Significantly different herbivory levels among the species (F = 6.29, p < .001) were observed. C. hirta showed significantly greater herbivory damage than P. hawaiiensis and P. cattleianum. P. albidus sustained an intermediate level of herbivory damage (Figure 2).
In terms of leaf traits, native species had significantly greater leaf area (t = 11.75, p < .001), leaf mass per unit area (LMA; t = -2.32, p = .021), and water content (t= -24.73, p < .001). Percent leaf damage was correlated with herbivory, and the only significant correlate of herbivory was LMA (r = -0.137, p < .001). As demonstrated by the low correlation coefficient, leaves with higher LMA had only slightly less herbivory damage.
DISCUSSION
There was a significant difference in arthropod abundance between the two sites. However, rather than finding higher diversity of arthropods within an invaded Hawaiian lowland wet forest (HLWF) as expected, we observed higher abundances of arthropods in the primarily native HLWF. One possible explanation for these findings is that there are fewer non-native arthropods to correspond with the non-native flora in the highly invaded HLWF. Alternatively, perhaps there are fewer native arthropods within the invaded site. However, it was not determined whether the arthropods were native or non-native in origin at either site. The invaded site may have fewer arthropods overall because introduced plant species have been found to reduce native animal species richness by altering ecosystem processes (Florens, Mauremootoo, Fowler, Winder, & Baider, 2010; Zagrobelny et al., 2008). A study of the butterfly community on the island Mauritius found that butterfly richness was much higher in weeded forests than in forests overwhelmed by weeds (Florens et al., 2010). Our results may indicate a similar effect in HLWF, which has important implications for native arthropod conservation.
This study found no differences in the number of orders observed between the different forest types (16 at the invaded site versus 17 at the native site). However, this study was unable to capture potentially greater diversity at lower taxonomic levels, such as families and genera. For instance, although Hymenoptera were observed in both types of traps, they were mainly represented by wasps in the color sticky traps and by ants in the pitfall traps. Additionally, many more spiders (Araneae) were found in pitfall traps within the native site than the invaded site (19 versus 2), but there were no such differences regarding the sticky traps. A previous study has shown that native HLWF have more open canopy and less concentration of leaf litter than invaded or mainland forests (Ostertag et al., 2009). This finding, together with the present study, suggests mostly native forest may provide a better forest floor hunting environment for predators, in contrast to an invaded HLWF.
In addition to higher diversity of arthropods, we originally predicted higher herbivory rates in the invaded HLWF. However, we actually observed higher overall levels of herbivory at the native site. In addition, it should be noted that the average leaf mass per unit area (LMA) was higher in native plant species compared to invasive species, a pattern that is supported by the literature (Penuelas et al., 2010) and suggestive of higher herbivory rates in invaded forests. Given the few differences in leaves’ LMA, area, and water content between native and invasive species and between sites, it is likely that factors other than plant traits control herbivory levels.
The differences in herbivory between sites could have been influenced by the fact that the native site has a more open mid- and understory that allows more sunlight to reach leaves. The plant vigor hypothesis predicts that herbivores are more likely to attack vigorously growing plants than those that are suppressed by shaded conditions (Hough-Goldstein & La Coss, 2012; Price, 1991). Thus greater light availability at the native site may contribute to the greater levels of herbivory observed in this study.
Higher herbivory rates in the native forest may also be linked to both the higher abundance and higher diversity of arthropods found in the native site compared to the invaded site (seven versus five; Figure 1). This raises many questions about the complexity of these differing HLWF ecosystems, their relationship with arthropod communities, and whether a primarily native HLWF may provide a more desirable habitat.
In short, the findings of this study support the importance of arthropod roles in herbivory within forest ecosystems. The biogeographic origins of the arthropods studied remain unclear, and individual species’ plant preferences are also unknown. It does not seem that the arthropods are selecting plants at the level of their origin; rather the difference is across forest types. In the long term, greater herbivory in the native forest may have cascading effects in trophic ecology and forest dynamics (Olofsson et al., 2004).
ACKNOWLEDGEMENTS
We wish to thank the Pacific Internship Programs for Exploring Science based at the University of Hawai’I (UH) at Hilo and grant # NSF REU 1005186, as well as Moana Ching, Noelani Puniwai, and Sharon Ziegler-Chong for coordination. We appreciate the Hawai’i Division of Forestry and Wildlife and Keaukaha Military Reservation for access to their lands and for permits to collect plant material. We also thank Dr. Laura Warman of the USDA Forest Service and Dr. Rebecca Ostertag of the UH Hilo Biology Department for their mentorship, as well as Nicole DiManno, Taite Winthers-Barcelona, and William Ray for their help in the field and laboratory.
REFERENCES
Alerding, A. B., & Hunter, R. M. (2013). Increased springtail abundance in a garlic mustard-invaded forest. Northeastern Naturalist, 20(2), 275-288.
Basset, Y. (1991). Influence of leaf traits on the spatial distribution of insect herbivores associated with an overstory rainforest tree. Oecologia, 87, 388-393.
Coley, P. D. (1983). Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs, 53, 209-229.
Cordell, S., Ostertag, R., Rowe, B., Sweinhart, L., Vasquez-Radonic, L., Michaud, J., & Cole, C. T. (2009). Evaluating barriers to native seedling establishment in an invaded Hawaiian lowland wet forest. Biological Conservation, 142, 2997-3004.
Florens, F., Mauremootoo, J. R., Fowler, S.V., Winder, L., & Baider, C. (2010). Recovery of indigenous butterfly community following control of invasive alien plants in a tropical island's wet forests. Biodiversity and Conservation, 19(14), 3835-3848.
Gillespie, R. G., & Roderick, G. K. (2002). Arthropods on islands: colonization, speciation, and conservation. Annual Review of Entomology, 47, 595-632.
Halaj, J., Ross, D. W., & Moldenke, A. R. (2000). Importance of habitat structure to the arthropod food web in Douglas-fir canopies. Oikos, 90(1), 139-152.
Hough-Goldstein, J., & La Coss, S. J. (2012). Interactive effects of light environment and herbivory on growth and productivity of an invasive annual vine, Persicaria perfoliata. Arthropod-Plant Interactions, 6, 103-111.
Hughes, R. F., & Denslow, J. S. (2005). Invasion by N-2-fixing tree alters function and structure in wet lowland forests of Hawaii. Ecological Applications, 15, 1615-1628.
Jacques, H. E. (1947). How to know the insects (2nd ed.). N.p.: C. Brown.
James, H. F., & Burney, D. A. (1996). The diet and ecology of Hawaii's extinct flightless waterfowl: evidence from coprolites. Biological Journal of the Linnean Society, 62, 279- 297.
Mascaro, J., Hughes, R. F., & Schnitzer, S. F. (2012). Novel forests maintain ecosystem processes after the decline of native species. Ecological Monographs, 221-228.
Minitab 16 Statistical Software (2010). [Computer software]. University of Hawai’i at Hilo, HI: Minitab, Inc. www.minitab.com.
Olofsson, J.,Hulme, P.E., Oksanen, L., Suominen, O. (2004). Importance of large and small mammalian herbivores for the plant community structure in the forest tundra ecotone.Oikos, 106(2), 324-334.
Ostertag, R., Cordell, S., Michaud, J., Cole, T. C., Schulten, J. R., Publico, K. M., & Enoka, J. H. (2009). Ecosystem and restoration consequences of invasive woody species removal in Hawaiian lowland wet forest. Ecosystems, 12, 503-515.
Penuelas, J., Sardans, J., Llusia, J., Owen, S. M., Carnicer, J., Giambelluca, T. W., & Rezendes, E. L. (2010). Faster returns on 'leaf economics' and different biogeochemical niche in invasive compared with native plant species. Global Change Biology, 16, 2171-2185.
Price, P. W. (1991). The plant vigor hypothesis and herbivore attack. Oikos, 62, 244-255.
R Core Team (2013). [Computer Software]. R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org.
Rasband, W.S. (2014). [Computer software]. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA: http://imagej.nih.gov/ij.
Wagner, W., Herbst, D. R., & Sohmer, S. (1999). Manual of the flowering plants of Hawaii (2nd ed.). Honolulu, HI: Bishop Museum.
Zagrobelny, M., Bak, S., & Moller, L. (2008). Cyanogenesis in plants and arthropods. Phytochemistry, 69(7), 1457-1468.
Zimmerman, N., Hughes, F. R., Cordell, S., Hart, P., Chang, H., Perez, D., & Like, R. K. (2008, March). Patterns of primary succession of native and introduced plants in lowland wet forests in eastern Hawai'i. Biotropica, 40(3), 277-284.