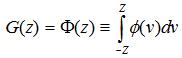Author: You Chan Kevin Chung
Institution: University of Pennsylvania
Date: March 2007
ABSTRACT
The Pediatric Exclusivity Act of 1997 was enacted to implement an additional six months of patent protection for a drug. As a result, if a manufacturer conducts studies of the drug on children, the drug company will be able to benefit by having an effective monopoly of the drug for an additional six months. While the aim of this provision was to increase the safety of drugs in the pediatric population, the pharmaceutical industry has been accused of exploiting the law for financial gain. The charge is that drug manufacturers are only using the act to gain an extended patent for its large selling drugs in order to profit at greater margins. The purpose of this study is to determine whether or not these accusations toward the pharmaceutical industry can be verified. It will analyze the major factors, answering why manufacturers would decide to run a trial of a drug in children or not. This study uses the Probit Model to statistically analyze these factors. The dependent variables, 1 and 0 were obtained from the Food and Drug Administration where 1 represented those drugs that have been granted 6 months extra exclusivity period and 0 representing those that are recommended yet not approved. The independent variables were analyzed in stepwise pattern and the marginal value for each of the 29 independent variables was calculated in six different sets. The sales component was the largest force in the exclusivity law when only 15 variables were included. However, when all 29 variables were assessed, Allergy and ADHD (Attention Deficit Hyperactivity Disorder) had the largest marginal values. This result suggests that the pediatric exclusivity act is not solely driven by the monetary incentive but it is just one of the many driving force in the law.
INTRODUCTION
Drug research on children has been an ongoing struggle in the pharmaceutical industry with debates on scientific, ethical as well as practical issues. While the number of drugs prescribed to children is at an ever increasing trend, only 20% to 30% of the drugs approved by the Food and Drug Administration are labeled for pediatric use (Meadows 2000). Other source states that about three fourth of the prescribed medication for pediatric use contain insufficient information (Blumer 1999). Children are not small adults and without the proper testing of the drugs on children, the safety of drugs that are widely used in the pediatric population can only be guesswork by resorting to existing labels for determining safety of the drugs. However, the drugs are labeled only for the adult population.
In order to promote the labeling of drugs for pediatric use and to give incentives for pediatric population testing, the Food and Drug Administration in 1997 passed the Modernization Act that provided six months of additional patent protection to the manufacturers who voluntarily conducted studies of drugs on children. The act is known to have received broad support from pediatricians and other public health advocacy groups stating that its benefit that outweigh its drawbacks.(Ward R America 2001) In January of 2002, the Best Pharmaceutical for Children Act was passed to reauthorized the act until 2007.
As a result of these following acts, it is estimated that in the past few years, the number of medications in pediatric clinical trials and the number of labels on children have increased tremendously. One study concludes that through Modernization Act, clinical trial on pediatric population has been stimulated allowing better understanding of drugs prescribed in pediatric medicine and improvement on the safety for children taking these drugs (Rosemary 2003). However, while this law showed great increase in the number of testing on children and the label change in drugs, it has been pointed out by many sources that the law is not being implemented as it should be. For example, there are arguments stating that financial gain is the sole driving force for the pharmaceutical company to do testing on children since it grants an additional 6 months exclusive patent period. While the Pediatric Exclusivity law was initially passed and showed success in terms of number of drugs tested on children, debate concerning child testing, its ethical issue as well as the practical difficulty has been ongoing. Ethically, because children are considered minors, the researches on children are scrutinized as the parents make decisions on behalf of their children. Prizes attached to the testing procedure often sway the parent's decision in giving permission for the manufacturer to conduct testing on the children. According to the American Academy of Pediatrics, there occurs a serious ethical question when payments are offered as incentive to the parents who are acting on behalf of minors to participate in research (Steinbrook 2002).
On the practical side of the matter, recruitment of children to do research in different age groups is difficult where it results in the testing not becoming credible enough with such small sample size. The FDA classifies pediatric patients into 4 categories by age group (newborns, infants, children, and adolescents).
Concerning the law itself, many critics have questioned the effect of the Exclusivity law. While on the surface, it shows great effectiveness in terms of the number of tests and label changes done on children, it is often misrepresented as it does not truly reflect the number of drugs that are used in children, emphasizing that pharmaceutical companies are just in it for the monetary prize of 6 months exclusivity. As it was pointed out in the Wall Street Journal, companies are first testing mainly their big selling drugs, where extended market exclusivity will award them large financial sums. Furthermore, Zimmerman points out the argument made by critics of the law stating the misuse of financial incentives. For example, Eli Lilly & Co. has won an addition 6 month patent with its Prozac product, adding an additional $831 million in revenue. However, the clinical study had been completed in 1995, two years prior to the passing of the law (Zimmerman 2001).
Furthermore, a more serious matter is addressed where the pharmaceutical companies do pediatric testing with drugs for conditions that are not common in juveniles. As the article points out, Bristol Myer's Glucophage for example, an adult onset diabetes medicine and Merck's Vasotec hypertension pill, while prescribed to children, are in an extremely small amount compared to the prescription for adult population. The Wall Street Journal points out that, according the market research firm IMS Health, while 24 million of Glucophase was prescribed in the United States in 2000, just 133,000 were written for pediatric patients. For Vasotec, a total of 11 million was prescribed while just 70,000 of them were prescribed by pediatricians. The testing done on both makers won them the extra exclusivity of patent length which brought in an estimated $1 billion in added revenue (Zimmerman 2001).
We hypothesize that the Pediatric Exclusivity Act of 1997 was indeed allowing pharmaceutical companies to further their profits. Our hypothesis was in concurrence with the Wall Street Journal, and was to test whether the bigger issue of using trials of drugs on children was solely to further the drug manufacturers' profits.
This paper specifically tackles the issue of the law, by statistically testing whether or not the claim that the pharmaceutical industry is using the provision for financial gain is true for every drug that has been approved and has been granted the extra patent protection. If it is the case that the financial source is the only driving force in the drug companies conducting tests on children, it suggests that the Pediatric Exclusivity Law presents problems in its effectiveness. However, if it is the case that the drug companies are not solely driven by monetary incentives, it serves the purpose of this law, getting labels on drugs that are widely used in the pediatric population. Furthermore, if the law's goal is being met with the pediatric drugs being tested and labeled, the ethical and practical issues could potentially be alleviated as the law is being used for the purpose of protecting and providing for the safety of children.
MATERIALS and METHODS
Constructing the Database
In order to interpret the Probit Model estimation, it is important to take the marginal effect as an interpretation. Therefore, out of all three tables tabulated, the marginal effect table is most important (table2). The marginal effect of the table constructed is essentially the probability of the specific independent variable being successful (in this case getting on list 1) if there was an addition of a unit in the mean of the specific independent variable. It must be pointed out that, this probability is under the condition that other variables are held constant at their mean. It is therefore important to focus on the marginal effect table and observe the movement of each independent variable as the set is increased from set 1 to set 6.
To perform a statistical test that could explain the chosen dependent variable, it was imperative to select necessary parameters for explaining the dependent variable. Because the statistical test aims to test for the efficacy of the Pediatric Exclusivity Law, a dependent variable was chosen by gathering datasets of those drugs that have either been approved by the FDA or will be approved if tests were done for that specific drug. Therefore, with the two sets of lists gathered from the FDA (FDA 1 2005; FDA 2 2001) it was labeled as list 0 and list 1 for the purpose of employing the Probit model.
For list 1, which was a list of pediatric exclusivity label changes (FDA 1 2005), the list was constructed with 3 components of independent variables (exclusivity date granted, product/manufacturer, indication). Out of the 3 components that were present, just 2 components were used for the statistical test (refer to database table attached). Furthermore, for the two components, product/manufacturer and indication, a 1 x 3 matrix was constructed for each product on the list. For the case of product/manufacturer, the three column spaces were divided into, large, medium and small, based on the employee size of the given pharmaceutical company determined from Hoovers A D&B Company (Hoovers March 2006). Companies with an employee size of 75,000 or more were labeled as large while medium was labeled for any company with the employee size between 25,000 and 75,000. Any company with employee size of less than 25,000 was categorized as a small sized company. Therefore, given the manufacturer of the specific drug, the matrix had one 1 in the 1x3 matrix while it had 2 zeroes in the columns that were not filled with 1. For list 1, the pediatric label changes that have already been granted, there were 102 observations which represented a 102 x 3 matrix for the company size component of the parameter. Similarly, for the indication component, a 102 x 3 matrix was constructed for list 1 by categorizing the indications as mild, moderate and severe. With the given description of indications, each drug was categorized by the 3 indication, 1 indication filled out as 1, while the other two as zeros.
For list 0, which is the list of drugs for which there is a list of drugs that the FDA has recommended for pediatric testing (FDA 2 2001) 417 x 3 matrices were constructed for both indication and company size. However, for the case of list 0, research on drug companies that produced the specific drug was necessary because the drugs were in the form of ingredient name, for company size component. Many times, a specific drug is produced by multiple companies. In such case just one company was selected. The criteria for choosing one company over another were based on company ownership (U.S. based vs. international) and variability of product. While the first criterion was straightforward, the second required some personal judgments. For example, for Benazepril, a drug that is used to treat hypertension, there were 8 different dosage forms with 3 different manufacturers. Four out of 8 were produced by Teva pharmaceutical company while the other 4 were produced by Ivax and Mylan. So in this case, Teva pharmaceutical was chosen as the manufacturer of Benazepril. The search for the manufacturer was done through many different websites, some pharmacy websites (such as Walgreen, CVS as well as websites that had detailed drug information (such as PharmInfoNet, Drugs). With this determination of pharmaceutical company manufacturer, a 417 x 3 matrix was constructed for each indication and company size. Therefore, the two lists in matrix form were combined to become a 519 x 3 matrix for each component,
Figure 3: This is the marginal effect result for set 3. Out of 14 independent variables that were included for the significant test, only sales component had a significant value with he marginal effect of 0.94%.
where xi,j is the indication, while zi,j is the company size while c is the constant and βi is the coefficient that is of interest. However, in the actual regression analysis, 1 component for each of the matrices was taken out to prevent the multicolinearity problem. With just 6 coefficients to explain the dependent variable, those unexplained by the components will be presented through the constant, C. In order to better understand the dependent variable, this initial construction was revised by including more components in the independent variable.
To expand the size of the independent variable, both indication and company size were combined to form a new matrix. Since both indication and company size were in a 1 x 3 matrix form, there were 9 combinations of indication and size to form a 1 x 9 matrix. Also, dummy variables were added for the specific purpose of singling out the company as well as the specific indication. Seven dummy variables were included for the company variables while 6 dummy variables were included for the indication components. The dummy variables were chosen in the manner that they were evenly represented (large medium and small) for size of company dummy variables as well as the indication (mild, moderate and severe) dummy variables. Referring to the list of "Top 10 drugs prescribed to kids without pediatric labeling"(Nordenberg 1999), conditions such as asthma, allergy, anxiety, depression, ADHD (attention deficit hyperactivity disorder) that corresponded to drugs that kids were prescribed most were included to test for significance (especially for indication dummy variables). Lastly, to assess whether or not the monetary incentive is one of the most significant components in explaining the dependent variable, a sale component was added by using the list of top 300 prescription of 2004 in U.S. (Top 300 2006). Therefore, the final equation that was constructed composed of,
Figure 4: This is the marginal effect result for set 4. 7 of the variables were found significant. As seen above, Pfizer dummy variable has the largest marginal effect value which shows that its component is most likely to be in list 1 (success) than any other components.
Probit Model
The model that was employed in this analysis was the Probit Model. Also known as the binary dependent Variable models (which also includes logit and tobit), the dependent variable y of this class of models can only take on two values, zero and one (Wooldridge 2003). In the case of the list that was created for the analysis, zero represented the list of drugs that was recommended by the FDA for pediatric testing. One represented the list of drugs that were already approved with labeling changes under the law. In order to briefly introduce the theory behind the binary dependent variable model, a mathematical approach is necessary as presented below (Eviews 2004).
Under the condition that we model the probability of observing a value of one as,
Pr(yi = 1|x1,β) = 1 F(-xi'β)
with the condition that F is a continuous function, strictly increasing which takes a real value and produces a value ranging from zero to one. The function F in this model (Cumulative distribution function of the standard normal distribution for the Probit model) determines the type of binary model. It follows that
Pr(yi = 0|x1,β) = F(-xi'β)
Given such a specification, an estimation of the parameters of the model is made using the method of maximum likelihood which is given by,
Table 1 shows the coefficients from the Probit regression analysis for the various pharmaceutical companies tested.
A binary model is often motivated as a latent variables specification. Under the condition that there is an unobserved latent variable yi* that is represented as,
yi* = xi'β + ui
Where ui is a random error. The dependent variable is determined whether yi* exceeds the threshold value,
yi = 1 if yi*> 0
yi = 0 if yi* 0
For the dependent variable in the analysis done on this paper, the above step of the determination of the dependent variable through threshold value was not necessary since it was defined by two lists that were present. Lastly, the function F that is employed in the Probit model analysis is the standard normal cumulative distribution function, expressed as an integral (Wooldridge 2003),
Figure 5: This is the marginal effect result for set 5. 9 of the variables were found significant. As seen above, Allergy has the largest marginal effect value which shows that its component is most likely to be in list 1 (success) than any other components.
Where ϕ(z) is a standard normal density,
ϕ(z) = (2π)-1/2exp(-z2/2)
Statistical Analysis using EVIEWS
Statistical analysis was carried on with the construction of the equation presented in the previous section. While the final equation consists of 29 independent variables, for the statistical analysis using the EVIEWS program, 6 different sets of tests were done by selecting different independent variables. This was primarily done to isolate independent variables sets for its movement of the coefficient as independent variables were included and excluded. Therefore, starting from set 1, which had just 6 components of size and conditions, independent variables were added sequentially until the 6th set had all 39 independent variables. Testing for coefficient and its significance was done for all 6 sets (see results section for table. Additionally, two marginal effect tables were constructed. Two simplified marginal effect calculations were done for comparison since both are simplified versions of a marginal effect. First, using the coefficient of the regression to represent dy/dx where this was multiplied by x/1 which was calculated through the average values of x. Thus, the marginal effect was calculated for each independent variable,
Figure 6: This is the marginal effect result for set 6 which had 30 independent variables included in the equation for Probit model estimation. Out of 30 variables, 11 of them were found significant. As seen above, Allergy has the largest marginal effect value which shows that its component is most likely to be in list 1 (success) than any other components.
The second marginal effect calculation comes by using the density function of the Probit model (Greene 2002).
Table 2 depicts the marginal effect values for the pharmaceutical companies tested using the Probit Model.
Lastly, with two tables tabulated for coefficients and marginal effect, the mean of each independent variables were tabulated as well.
RESULTS
The marginal values for each independent variable in each of the six different sets were calculated and tabulated. In set 1, the Mild and Moderate component have the largest marginal effect values. With only 6 components included in the independent variable, 4 were significant. In set 2, the Sale component has the largest marginal effect value which signifies that sale is most likely the driving force of the success (list 1). For set 3, out of 14 independent variable that were included for the significance test, only the Sales component had a significant value, with a marginal effect of 0.94%. Set 4 demonstrates that 7 of the variables were found to be significant. As seen above, the Pfizer dummy variable has the largest marginal effect value. Nine of the variables were found to be significant for set 5. As shown, Allergy has the largest marginal effect value. Lastly, for set 6, the marginal effect result for the 30 independent variables included in the equation for the Probit model estimation is shown. Out of the 30 variables, 11 were found significant. Again, Allergy has the largest marginal effect value.
Set 1 (Size & Indication)
article_926_order_0
Set 1 consists of 6 variables, 3 variables from the size and 3 from the indication. However, in the actual coefficient test, 1 from each component was taken out to avoid the multicollinearity problem. These specific components' coefficients would be evident and picked up by the constant C in the independent variable in the Probit model. The result shows that all of the variables are significant except for the medium component, which represents the medium sized pharmaceutical (companies with employee size between 25,000 and 75,000). Drugs that treat both mild and moderate symptoms came out to be significant. Looking at the marginal effect for both of these independent variables, it shows its marginal effect when one unit of increase is made to the mean. Since two marginal effects were calculated, and since it was shown that the ranks are the same (refer to table 3) just one of the two coefficients, Xk will be discussed. Table 2 suggests that with one unit of increase of the mean for mild (0.0829) and moderate (0.2987), the probability of getting on list 1 will increase by and 0.004 and 0.002 respectively. Looking at the other two significant values, the constant and small, both have a negative marginal value, suggesting a reverse direction of the probability a decrease when the mean of each independent variable was increased by one unit. It is noted that the constant in this specific group is a representation of both the large company size and severe symptom drugs combined. While the two cannot be separated in this specific set, it shows that the combination of the two results in negative marginal effect with the marginal coefficient of -0.0060. The first set signifies a Mild drug being most likely to be on the list 1 since it has the largest marginal effect ie) with the one unit of increase in the mean, it has the largest probability of being on list 1. In fact, a Mild drug has twice the probability of the increase as compared to the moderate drug.
Set2 (Size & Condition and Sale)
article_926_order_1
article_926_order_13
From set 1, one more component was added to set 2 for the test of the Probit model. The result shows that the Mild and the Moderate independent variables are no longer significant. The sales component that was added however is significant to the 1% confidence interval. The Company size of the small and the constant variable that is a combination of both the large company size and severe symptom drug is significant to the 1% confidence interval. In terms of marginal effect, sale shows the greatest probability with 0.008 which signifies the probability of getting approved when an additional unit is added on the present mean 0.22736. The constant value as well as the small size company variable shows a negative marginal effect which is consistent with set 1.
Set 3 (Size, Condition, Sale, Interactions)
article_926_order_8
In set 3, interaction components were added from set 2. The interaction basically combined the size and the condition component which resulted in the addition of 9 combinations of interaction components. Looking at the result, only the sales component is significant when the marginal effect is 0.00937. This shows that in this particular set, the sale variable is the only explainable variable for making it to list 1. Thus, this set suggests that financial reward is the only driving force behind the pediatric exclusivity law. However, the analysis does not stop here since it is necessary to add more variables to the independent variable for explaining the dependent variable.
Set 4 (Size, Condition, Sale, Interactions, Firm dummies)
article_926_order_9
Firm dummies were included in this set and out of 7 firm dummies that were included, 6 of them show significance. (The only non significant firm being Abott laboratories). In contrast to set 3, the dummy variables of the company explain the dependent variable better than previous sets with more variables being significant. The largest marginal effect in set 4 is the Pfizer dummy with the value of 0.021 which suggests a 2.1% increase in the probability of making it to list 1 if the mean were increased by 1 unit with others kept constant. This suggests that Pfizer is more likely to be on the list under the same condition of other variables increasing their mean by 1 unit. The Pfizer dummy is followed by the Glaxosmith dummy with the marginal coefficient of 0.018 which suggests a 1.8% increase of the probability if the mean were increase by 1 unit. The sale variable is ranked 3rd in the set 4 list with 0.017, not far below the two dummy variables preceding the sale variable. Interestingly, in set 4, no independent variables, neither condition nor interactions, are significant. Also, while the Abott dummy was not significant, the Mylan dummy was negatively correlated with the dependent variable, showing an inverse marginal effect in list 1. This negative value of the marginal coefficient suggests that Mylan's chance of getting onto list 1 is decreased when the mean is increased by 1 unit. This also suggests that if the mean were decreased by 1 unit, then the Mylan dummy would have a positive marginal effect, which is quite counterintuitive.
Set 5 (Size, Size, Condition, Sale, Interactions, Firm dummies, Condition dummies)
article_926_order_10
Set 5 included 7 condition dummies that represented a wide range of symptoms (see methods for details). The result shows that while all the significant components are carried over from set 4 except for the Bsquibb dummy, two condition dummies are found to be significant. Allergy and asthma, which is found to be significant, showing that they are significant in the 5% confidence interval. Also, the constant term turns out to be significant to the 10% significance level where it represents the Conditionothers, large and severe components combined. Knowing that the previous set, set 4, had an insignificant constant which represented the large and the severe component, this significance in set 5 therefore represents the significance of Conditionothers, which are conditions other than the 7 conditions that are represented in the independent variable. The greatest marginal effect is the Allergy dummy, which is followed by the Pfizer dummy. This suggests that while set 4 showed Pfizer being the greatest marginal effect dummy, adding another independent variable could potentially give new insight in better explaining the dependent variable. However, for other independent variables, the ranking of set 4 is carried over as the rank of set 4 with the exception of the Mylan dummy, followed by the Allergy dummy in the rank for set 5. (The Bsquibb dummy is the only dummy that is not significant, which is not included in set 5 that is included in set 4.) Followed by the rank of set 4, the 7th, 8th and 9th largest marginal effect coefficients are the Asthma, Mylan and the constant C variables. These marginal effects are negative, indicating negative movement in probability if mean were increased by one unit for these respective independent variables.
Set 6 (Size, Size, Condition, Sale, Interactions, Firm dummies, Condition dummies w/ condition others)
article_926_order_11
Taking out the Cold component to be represented in the constant term, the Condtionother component was included in the independent variable. As picked up in set 5 with the Conditionother component being significant, the value shows significance. In terms of marginal effect, the Allergy dummy is the greatest with the value of 0.034 which is consistent with set 5. This implies that if the mean of Allergy dummy were increased by 1 unit then the probability of it being included in list 1 increases by 3.4%. What is most interesting in list 6 is that the two condition dummies take the spot of rank 2 and 3 for the marginal effect. ADHD and HIV are followed by the Allergy dummy in the marginal effect coefficient. The respective values are 0.028 for ADHD and 0.024 for HIV which suggests a 2.8% and 2.4% increase of each variable's likeliness to be on list 1 if their respective means were increased by 1 unit, keeping others constant. The Pfizer and Glaxosmith dummies take the spot of 4 and 5 for the ranking followed by the sales dummy which shows that in set 6, financial benefit is not the number 1 driving force that enables the drug to be listed in list 1. It is interesting to find out that interaction the dummies as well as the condition dummies were not significant throughout the sets, perhaps because there are no real forces under these two components that drives the pharmaceutical companies to do testing on children. What is interesting to point out is that Allergy dummy has the best marginal effect which is one of "Top 10 drugs prescribed to kids without pediatric labeling" (Nordenberg 1999). Two out of top three marginal effect coefficients (allergy and ADHD) corresponded to drugs that kids were prescribed to most. These were ranked first and second in set 6, demonstrating that the tendency of the tests to be done on children is greatest for these two dummies.
The tables constructed for the analysis with the 6 different sets is provided below. The tables provide a comprehensive overview of the database constructed for the statistical analysis for all the pharmaceutical companies tested. Again, Table 2 provides the most meaningful interpretation of the results, as it gives the probability of the specific independent variable being getting on list 1 if there was an additional unit in the mean of the specific independent variable.
article_926_order_12
article_926_order_13
article_926_order_14
article_926_order_15
DISCUSSION AND CONCLUSIONS
We hypothesized that the exclusivity law allowed pharmaceutical companies to conduct tests on children solely for financial gain. However, the results of set 6 in the analysis reveals that the pediatric exclusivity law is not being solely driven by the monetary aspect which grants the companies 6 months extra patent length. The sales dummy is ranked actually 6th out of 11 significant components which also verifies that it is not the largest force in the overall behavior of this law. As the result section shows, the 6 sets of the result are worth elaborating on its significance. In set 1, where only 6 independent variables were included in the regression, it appeared that the dependent variable was best explainable with mild and moderate condition where the marginal effect was the greatest with 0.004 and 0.002 respectively. While this result seemed plausible, once a Sales variable was included in set 2, the two variables in set 1 that were dominant no longer became significant. Instead, the Sales component had the greatest marginal effect, demonstrating that financial incentive is indeed the driving force of the Pediatric Exclusivity Act of 1997. Furthermore, set 3 further confirmed this suggestion as it was shown to be the only significant variable out of 15 independent variables. If the significance test was stopped at this point, it would have been possible to conclude that the Pediatric Exclusivity Act is not being implemented as it should and it would confirm the claim that the Wall Street Journal (Zimmerman 2001) made in the article about pharmaceutical companies solely driven by the financial reward. The first 3 sets showed that the sale component is dominant once the independent variable was introduced, whereas other variables were left being insignificant. Sets 4 to 6 introduced the firm dummies as well as condition dummies for better estimate for the Probit Model. With the independent variable ranging from 22 to 28, a better understanding of the Pediatric Exclusivity Act was made possible. Set 4 represents the regression of size, condition, sale, interactions and firm dummies variables. The result shows that the Pfizer dummy has the largest marginal effect followed by Glaxosmith dummy. Both being in the large company category (employee size of more than 75,000) this set showed that the larger the size of the pharmaceutical company, the more likely it is for it to conduct testing on children as the marginal effect is the greatest. The sales component was followed by these two variables which suggested that the financial incentive is still there. All the firm dummies except the Abott dummy came out to be significant which shows specific companies do have an edge as their probability of getting on list 1 is larger than the other companies.
Out of all the sets, sets 5 and 6 are most interesting and most significant in terms of the statistical analysis done. As it could be seen from table 5 and table 6, including the condition dummies makes a significant change in the result. The Allergy dummy being the significant dummy with the largest marginal effect refutes the conclusion made in set 3 and 4 where it was shown that perhaps the sales as well as company size were the dominant component in explaining the Pediatric Exclusivity Law. Especially when Allergy (Nordenberg 1999) is one of top 10 most prescribed drugs in pediatric population, both set 5 and set 6 show that it is not only the financial incentive that is the driving force for this law. Although it is true that the sales variable is ranked 4th and 6th respectively in set 5 and set 6 for marginal effect (refer to table 3 for ranking of marginal effect) it shows that it is not the sole driving force in the law. In set 5, while the Allergy dummy has the greatest marginal effect, it is followed by 4 companies and the sales variable. This perhaps shows that financial incentives as well as the specific company does play a large role in being approved for exclusivity. It must be mentioned however that out of 4 companies set 5 that has positive marginal effect are composed of 2 large 1 medium and 1 small company. So there is no dominant size of company with respect to their marginal effect. This could possibly suggest that it is not necessarily the size of the firm but the name brand or some other source that enables them to have an edge over the others.
Figure 6 which shows that the marginal effect of set 6 differs from set 5 in that rather than using the cold dummy for the condition, the Conditionothers dummy was used. The results show that the Allergy dummy is once again the most dominant factor followed by ADHD which is also in the top 10 list of most prescribed drugs for Pediatric population (Nordenberg 1999). The Pfizer dummy has the largest marginal effect out of the firm dummies where it comes 4th in terms of the magnitude of the marginal effect. The sales component has the 6th largest marginal effect which again refutes the set 3 result. It is interesting to see that in both sets 5 and 6, the interactions dummies are not significant. Also, the negative marginal effect which is somewhat counterintuitive as it suggests that when the component's mean is increased by one unit, its probability of being "successful" (being on list 1) is decreased. This could possibly be the crowding out effect where it prevents certain players to enter. In set 5, the negative marginal effect variables were Asthma and Mylan, as well as the constant variable. The constant variable here represents the Conditionothers dummy, and medium and small sized companies combined. In set 6, the constant C and the Mylan dummy showed negative marginal effect. The constant variable in this case included the combination of both Cold dummy, Large and severe variables combined.
As the results show, Wall Street Journal's claim of the law being wrongfully implemented was not statistically proven in this paper. While set 1 to set 3 did indeed show that the sales component had the largest marginal effect values, which signifying that this was the greatest driving force in explaining the law and its implementation. With more independent variables included in the equation, it was apparent that the sales component was not the component with the largest marginal effect value. Likewise, even though 30 independent variables were included in the equation, it is likely that with more variables included in the equation, the marginal effect might change when the Probit Model estimation is carried through. Therefore, for the future consideration, including relevant independent variables that were not included in this paper could be something that is worth of doing.
The database was constructed using many different public resources. For example, drug companies that were used in each drug were searched through different sources online that showed its manufacturer. Compromise needed to be made when there were multiple manufacturers where it could have resulted in a bias in the database construction. While doing this research, it was found that NIH (National Institute of Health) is currently compiling a database on pediatric medication use, entitled "Frequency of Medications Usage in the Pediatric Population: A Comprehensive Data Analysis and Report Funding Source," which could be useful when one considers a similar direction in the future.
It should be mentioned that it was recommended to include several peer reviewed publications relating to the Pediatric Exclusivity Act. However, due to the novelty of this topic the availability of the literature was lacking. In fact, one study that was done to quantify the dissemination of the result of studies conducted under this law into the peer review literature concludes that while the Pediatric law has been successful in encouraging clinical trials on children, these results has been limited in the realm of peer reviewed literature. (Benjamin 2006) Therefore, while this paper could have been more scientifically cogent with reference to peer reviewed literatures, due to its novelty of the subject matter and its topic being specific within the Pediatric Exclusivity law, sources on the subject matter were limited. Further revision and studies would enhance the paper by incorporating peer reviewed literature.
Lastly, it must be mentioned that the test showed that specific drug companies have an edge over another in getting the drugs approved for 6 months exclusivity. From set 6, it was shown that Pfizer had the largest marginal effect values which suggests that Pfizer will most likely to be successful' compared to other company dummies. GlaxoSmithKline followed Pfizer which was followed by AstraZeneca and Merck. Given the fact that the 4 significant firm dummies are composed of 2 large 1 medium and 1 small company, it suggests that it is not necessarily the size of the firm but the name brand or some other sources that enables them to have an edge over the others. For future studies, it would be interesting to study these specific firm dummy components and study why one company has more advantage over another in the marginal effect values.
ACKNOWLEDGEMENTS
I would first like to thank Kwanjeong Educational Foundation of South Korea for supporting me financially for the 4 years of my undergraduate education. Without their support it would not have been possible for me to have the education that I had here at the University of Pennsylvania. The fellowship signified their trust in my potential and my ability which enabled me to discover my passion and further thrive in the field. I sincerely thank their support.
I want to take this opportunity to thank Professor Guy David in helping and assisting me throughout the year in completing this thesis. Without his generosity and his kindness I would not have been able to complete this thesis.
I want to thank Ms. Danianne Mizzy, Assistant Head of the Engineering Library for all the help she has provided in the initial stage of my research. As a novice in research, she was more than willing to help me in finding the research materials.
Lastly, I would like to thank my parents for all the things they have done for me in my life.
REFERENCES
Benjamin, Jr, Daniel K. M.D., PhD (September 13, 2006) "Peer-Reviewed Publication of Clinical Trials Completed for Pediatric Exclusivity" The Journal of American Medical Association Vol 296, No. 10.
Blumer, Jeffrey L. PhD, M.D., (September 1999) "Off-label Uses of Drugs in Children." (1999) Pediatrics Vol. 104 No. 3
CVS, www.cvs.com (March 15, 2006)
Drugs, www.drugs.com (March 15, 2006)
Eviews, Quantitative Micro Software (2004) Eviews5 User's Guide. 606.
FDA 1, Pediatric Exclusivity Labeling Changes, as of November 23, 2005 (March 3, 2006) (http://www.fda.gov/cder/pediatric/labelchange.htm).
FDA 2, Update of List of Approved drugs for which additional pediatric information may produce Health Benefits in the Pediatric Population (May 20, 2001) (http://www.fda.gov/cder/pediatric/peddrugsfinal.htm)
Greene, William H (2002) Probit Model interpretation. Econometric Analysis, Fifth edition.
Hoovers, www.hoovers.com (March 15, 2006)
Meadows, Michelle (March 3 2006)"Drug Research and Children" recent studies are providing important new information about drug safety and effectiveness for children. Pediatricians say it's about time. U.S Food and Drug Administration, FDA Consumer Magazine (http://www.fda.gov/fdac/features/2003/103_drugs.html).
Nordenberg, Tamar, (May-June, 1999) Top 10 Drugs Prescribed to Kids Without Pediatric Labeling, (1994) data based on research firm IMS America Ltd. (March 3, 2006) (http://www.fda.gov/fdac/features/1999/399_kids.html)
PharmInfoNet, http://pharminfo.8media.org/drugdb/dbgn_mnuf.html (March 14, 2006)
Rosemary Roberts, MD (August 20, 2003) "Pediatric Drug Labeling; Improving the Saftey and Efficacy of Pediatric Therapies" The Journal of American Medical Association Vol 290, No. 7.
Steinbrook, Robert, M.D (October 31, 2002) "Testing Medications in Children" Health Policy Report. The New England Journal of Medicine Journal 18: 1462-1470.
Top 300, The list of top 300 prescriptions for 2004 by number of US prescriptions dispensed. (March 1, 2006) (http://www.rxlist.com/top200.htm)
Walgreen, www.WalGreens.com (March 15, 2006)
Ward R America Academy of Pediatrics testimony before the United States senate Committee on Health, Education, Labor and Pensions; May 8, 2001. (November 30, 2006) (http://www.aap.org/advocacy/washing/testimonymay8thbward.htm)
Wooldridge, Jeffery M. (2003) Introductory Econometrics, A modern approach 2E. 553 Chap 17.
Zimmerman, Rachel (February 5, 2001) "Child Play: Pharmaceutical Firms Win Big on Plan to Test Adult drugs on kids-By doing inexpensive trials, they gain 6 more months free from generic rivals." The Wall Street Journal