Jennifer Kovach 1, Chloe Mazza1, Elle McGregor1, Casidy Shade1, Emily Violette1, Lara D. LaDage1
1 Division of Mathematics and Natural Sciences, Penn State Altoona, 3000 Ivyside Park, Altoona, PA 16601
Abstract
Animal-based fertilizers have been shown to be more environmentally friendly than commercial fertilizers. Cricket frass in particular contains sufficient nutrient contents and may be a viable natural alternative to chemical fertilizers, particularly for small scale use. Due to crickets’ small size, it would be advantageous to understand how to increase frass production. However, there is no study examining factors that may modulate frass production. As such, our study was designed to determine if olfactory stimulation can change frass production in banded crickets, Gryllodes sigillatus. Sweet orange and peppermint essential oils (Natrogix, Irvine, CA) were utilized as the stimuli in the cricket enclosures and mass of cricket frass (g) was recorded over 15 days. We predicted that the sweet orange oil would increase frass production, while the peppermint oil would decrease production, due to sweet orange being an attractive odor for the crickets and peppermint as a repellant odor. We found that frass production increased between the beginning and end of the experiment, but that essential oil treatment had no statistically significant effect on frass production or the interaction between time and essential oil treatment. Therefore, we conclude that there is no association between orange and peppermint essential oil exposure and frass production over 15 days in banded crickets, G. sigillatus.
Keywords: cricket frass, olfactory stimulation, natural fertilizer, essential oil
Author Summary
Our goal in this study was to examine the effects of odor on frass, or feces, production in crickets. Cricket frass contains nutrients that can serve as a fertilizer for soil, so we were interested in determining if there is a way to increase frass production. The results of our study showed that neither the sweet orange nor peppermint scent increased frass production among the crickets. The findings of this study may be significant for commercial cricket vendors, as these companies could generate income from frass converted to fertilizer instead of sending it to waste.
Introduction
Fertilizer is widely used in agriculture to increase nutrient concentrations in the soil and enhance crop and plant production. However, excess phosphorus and nitrogen from commercial fertilizers can have negative effects on the environment, such as eutrophication of water sources via runoff (Hart et al. 2004). Animal based fertilizers, such as manure, are a more natural and organic alternative to commercial fertilizers and can add needed nutrients to the soil (Goulding et al. 2008). While manure is appropriate for large plots of land, insect waste can be beneficial for smaller areas like gardens. Insect waste, or frass, has been shown to be agriculturally beneficial, and frass-based fertilizers have had promising effects on both crop yield and soil health (Beesigamukama et al. 2022). When comparing common insects like crickets, silkmoths, caterpillars, mealworms, desert locusts, African fruit beetles, and rhinoceros beetles, crickets had the highest nutrient concentration in their frass (Beesigamukama et al. 2022). Because of this, multiple companies have already begun to sell frass as a fertilizer (Houben et al. 2020). This may be of particular interest for commercial insect vendors as, rather than sending frass to waste, they could monetize cricket frass as a natural fertilizer. If frass production can be manipulated by various environmental factors, such as odor, to increase the amount of frass produced, more natural fertilizer can be produced and sold.
Odors act as stimuli for insects and may be advantageous in modulating frass production. Insects detect scent with olfactory receptor neurons in their antennae (Sato and Touhara 2008). Odor molecules, after making contact with the olfactory receptor neurons, are converted to an electrical impulse that is sent to the insect's brain (Sato and Touhara 2008). Processing of chemical odorants has been shown to modulate insect behavior, including attraction to conspecifics and identification and evaluation of food sources (De Bruyne and Baker 2008), as well as changes in physiology (Krzyzowski et al. 2020). Furthermore, crickets are capable of retaining olfactory memory for their entire lifetime, allowing them to associate scents with positive or negative experiences (Matsumoto 2022). While insects utilize chemical odorants to organize their physiology and behavior, it is less clear how olfactory stimulation can modulate consumption and subsequent waste production.
In this study, we aimed to understand whether olfactory stimulation and frass production are linked. The benefits of frass in agriculture are understood independently from the olfactory pathway in insects and there is no research linking olfaction in insects as a stimulus for frass production. As such, the goal of this experiment is to gain a better understanding of the effects of odors on frass production in crickets. In this study, we utilized essential oils derived from plant extracts as our odor stimulants, as plants serve as a primary food source for insects. Plant-emitted volatile organic compounds also comprise a large part of an insect’s odorscape and can alter physiology and behavior (Conchou et al. 2019; De Sousa et al. 2015). Volatile organic compounds released from plants attract insects to consume them, leading to frass production (Conchou et al. 2019; De Sousa et al. 2015). Crickets also have the ability to distinguish between essential oils (Matsumoto and Mizunami 2000). We hypothesized that olfactory stimulation in the form of sweet orange odor and peppermint odor would alter food consumption, reflected by changes in frass production. We predicted that frass production would increase when crickets were exposed to the sweet orange oil. Sweet orange is an attractive odor in crickets (Volpe et al. 2020), likely because oranges contain glucose, the most widely used sugar by insects for energy production (Vaulont et al. 2000). We predicted that peppermint oil, an odor shown to be repellant in other insects (Diaz-Montano and Trumble 2013), may decrease or have no effect on frass production in comparison to the control.
Materials and Methods
This experiment took place at the Penn State Altoona laboratories. While insect invertebrates are exempt from IACUC approval and are not covered in the Guide for the Care and Use of Laboratory Animals (National Research Council 2010), all care and use of crickets followed the best practices recommended in Frye (1992). Adult Banded crickets, G. sigillatus, were obtained from a commercial vendor (www.ghanns.com) and were initially housed in a 10-gal tank with cardboard egg crates as a hide. Commercial dry cat food and water were provided ad lib.
For the experiment, 150 crickets were randomly chosen from the tank and randomly assigned to three treatment enclosures (between 33.65 x 19.69 x 20.96 cm and 38.74 x 24.13 x 25.4cm). Each enclosure had cardboard egg cartons as a hide. Commercial cat food, 39.550g, was put into each container on the first day, along with Fluker’s Cricket Quencher as a water source; both food and water were placed in plastic containers.The food did not need to be replenished during the experiment, while the water source was replaced as needed. Fifty crickets were put in each container. In each container, a cotton ball was taped to each of the top four corners of the container (20cm high), precluding the crickets from direct contact with the cotton ball. In container one, three drops of peppermint essential oil (Natrogix, Irvine, CA) were put on each of the cotton balls. In container two, three drops of sweet orange essential oil (Natrogix, Irvine, CA) were put on each of the cotton balls. In container three, no oil was used on these cotton balls, to serve as a control.
Frass was collected every third day and production was assessed over 15 days. Every third day, the frass was collected via toothbrush, swept into weigh boats, and weighed. The mass of the frass was measured using an electronic scale and recorded. The number of living crickets in each container was also recorded and three drops of the orange or peppermint oils were added to their respective cotton balls to maintain the scent.
Frass production per cricket was calculated for the beginning and end of the experiment. We used a repeated measures analysis of variance (ANOVA) to analyze the effects of essential oil treatment and time point on average frass production. All analyses were conducted with SPSS for Windows, v. 27 (IBM Corp.) and results were considered to be statistically significant if p ≤ 0.05.
Results
Box’s test of equality of covariance matrices indicated conformation to equality of covariances (p = 0.658).
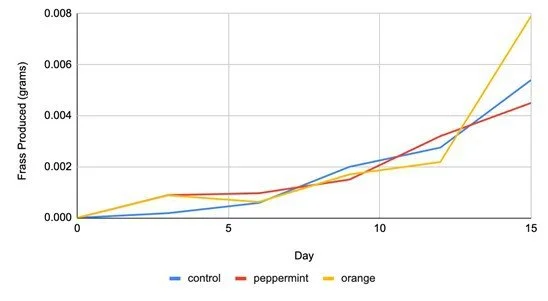
We found that frass production per cricket increased between the beginning and end of the experiment (F1,3 = 11.24, p = 0.044; Figure 1)
but that essential oil treatment (F2,3 = 0.1386, p = 0.8759)
and the interaction between time and essential oil treatment had no statistically significant effects on frass production (F2,3 = 0.14, p = 0.8747).
While we did see an increased mortality over time (F1,6 = 74.941, p < 0.0110),
mortality did not vary over time based on treatment (F2,6= 2.00, p = 0.695).
Figure 1. The average frass produced per cricket (g), in each experimental group, over a 15-day period.
Discussion
Average frass production increased between the beginning and end of the experiment among all treatment groups (Figure 1). However, there were no statistically significant effects of essential oil treatment or the interaction between time and essential oil treatment on frass production. Therefore, we reject our hypothesis that olfactory stimulation in the form of odors of sweet orange and peppermint may change frass production.
Distinguishing between olfactory stimuli may be particularly important for consumption, as insects utilize odor in locating food sources. Smell is a key sense used when navigating towards food (Zjacic and Scholz 2022). Previous studies have demonstrated that crickets and other insects are attracted to and differentially consume food based on olfactory information (Matsumoto and Mizunami 2000; Decker et al. 2007). These studies utilized both vanilla and peppermint essential oils to assess their effects on preference tasks. It was found in the first study that crickets initially preferred vanilla over peppermint, as the vanilla was associated with water and peppermint with saline solution. However, after the associations switched and the peppermint was paired with water, the crickets’ preference also switched (Matsumoto and Mizunami 2000). The second study had similar results, with cockroaches showing preference for peppermint when associated with a positive stimulus over the vanilla that was associated with a negative stimulus (Decker et al. 2007). These studies indicate that insects have the ability to distinguish between scents when they are paired with neutral or aversive gustatory stimuli. Along with an understanding of the insect olfactory system, the results of the aforementioned studies taken together suggest that our crickets had the ability to distinguish between sweet orange and peppermint essential oils. As such, our lack of significant results based on olfactory treatment was likely not due to a lack of ability in distinguishing odors; rather, the crickets may have either become habituated to the odors or did not place value in these odors. While the aforementioned studies found that essential oils could be used as an attractant when paired with other stimuli, in contrast, other studies have found essential oils to be an insect deterrent. A study done on Argentine ants, Linepithema humile, and red imported fire ants, Solenopsis invicta, revealed that 24 hours of continuous exposure to peppermint, tea tree, and citronella essential oils caused significant mortality in both insect groups (Wiltz and Suiter 2007). However, the insects in that study were in direct contact with the essential oils, whereas the crickets in our study were not. In our study, we did not observe a difference in mortality rate between the control and experimental groups, suggesting that direct exposure to essential oils may be the intermediary between essential oils and increased mortality rate. Alternatively, the increase in mortality in the other study may be due to the potency of the odorant, rather than contact with the oil. It may be that increased essential oil potency due to increased proximity could lead to increased mortality. Future studies may also focus on the potency of the odorant as a determinant of frass production.
While we did not find a difference among oil treatments in this study, there were potential limitations to our study that may obscure differences, if they existed. First, ambient temperature was not highly controlled and fluctuated between 21°C and 23°C. While the preferred microclimate for G. sigillatus is unknown, a previous study found a similar species, Gryllodes supplicans, to have greatest survival and reproduction at temperatures of around 29°C. (Smith and Thomas 1988). Assessing and housing crickets with appropriate microclimates may decrease mortality, possibly producing different results. Also, we constrained our experiment to 15 days; a longer duration could potentially change resultant frass production. Finally, we did not vary potency or proximity of the essential oils; a previous study demonstrated a difference in mortality with the use of essential oils, therefore our results may not be extrapolated to different contexts where the potency of the oils vary. By further investigating how to modulate frass production, an association between olfactory stimulation and increased frass production will likely be revealed.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank Cori Biddle, Penn State Altoona's assistant librarian, for help with literature and journal searches and Mark Oswalt, the Biology lab manager, for helping to set up the experiment.
References
Beesigamukama, D., Subramanian, S. and Tanga, C. M. (2022). Nutrient quality and maturity status of frass fertilizer from nine edible insects. Scientific Reports, 12(7182), available: https://doi.org/10.1038/s41598-022-11336-z
Conchou, L., Lucas, P., Meslin, C., Proffit, M., Staudt, M. and Renou, M. (2019). Insect odorscapes: From plant volatiles to natural olfactory scenes. Frontiers in physiology, 10(972), available: https://doi.org/10.3389/fphys.2019.00972
De Bruyne, M. and Baker, T. C. (2008). Odor detection in insects: Volatile codes. Journal of Chemical Ecology, 34(7), 882-897, available: https://doi.org/10.1007/s10886-008-9485-4
De Sousa, D., Hocayen, P., Andrade, L. and Andreatini, R. (2015). A systematic review of the anxiolytic-like effects of essential oils in animal models. Molecules, 20(10), 18620-18660, available: https://doi.org/10.3390/molecules201018620
Decker, S., McConnaughey, S. and Page, T. L. (2007). Circadian regulation of insect olfactory learning. Proceedings of the National Academy of Sciences, 104(40), 15905-15910, available: https://doi.org/10.1073/pnas.0702082104
Diaz-Montano, J. and Trumble, J. T. (2013). Behavioral respnses of the potato Psyllid (Hemiptera: Triozidae) to volatiles from dimethyl disulfide and plant essential oils. Journal of Insect Behavior, 26(3), 336-351, available: https://doi.org/10.1007/s10905-012-9350-8
Frye, F.L. (1992). Captive invertebrates: a guide to their biology and husbandry. Malabar: Krieger Publishing Company.
Goulding, K., Jarvis, S. and Whitmore, A. (2008). Optimizing nutrient management for farm systems. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1491), 667-680, available: https://doi.org/10.1098/rstb.2007.2177
Hart, M. R., Quin, B. F. and Nguyen, M. L. (2004). Phosphorus runoff from agricultural land and direct fertilizer effects: A review. Journal of Environmental Quality, 33(6), 1954-1972, available: https://doi.org/10.2134/jeq2004.1954
Houben, D., Daoulas, G., Faucon, M.-P. and Dulaurent, A.-M. (2020). Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Scientific Reports, 10(1), available: https://doi.org/10.1038/s41598-020-61765-x
Krzyżowski, M., Baran, B., Łozowski, B. and Francikowski, J. (2020). The effect of Rosmarinus officinalis essential oil fumigation on biochemical, behavioral, and physiological parameters of Callosobruchus maculatus. Insects, 11(6), 344, available: https://doi.org/10.3390/insects11060344
Matsumoto, Y. (2022). Learning and memory in the cricket Gryllus bimaculatus. Physiological Entomology, 47(3), 147-161, available: https://doi.org/10.1111/phen.12387
Matsumoto, Y. and Mizunami, M. (2000). Olfactory learning in the cricket Gryllus bimaculatus. Journal of Experimental Biology, 203(17), 2581-2588, available: https://doi.org/10.1242/jeb.203.17.2581
National Research Council. (2010). Guide for the care and use of laboratory animals.
Sato, K. and Touhara, K. (2008). Insect olfaction: Receptors, signal transduction, and behavior. Springer Berlin Heidelberg, 47, 121-138, available: https://doi.org/10.1007/400_2008_10
Smith, R. and Thomas, W.B. (1988). Southwestern distribution and habitat ecology of Gryllodes supplicans. Bulletin of the Entomological Society of America, 34, 90-186.
Vaulont, S., Vasseur-Cognet, M. and Kahn, A. (2000). Glucose regulation of gene transcription. Journal of Biological Chemistry, 275(41), 31555-31558, available: https://doi.org/10.1074/jbc.r000016200
Volpe, H. X. L., Zanardi, O. Z., Magnani, R. F., Luvizotto, R. A. G., Esperança, V., Freitas, R. D., Delfino, J. Y., Mulinari, T. A., Carvalho, R. I. D., Wulff, N. A., Miranda, M. P. D. and Peña, L. (2020). Behavioral responses of Diaphorina citri to host plant volatiles in multiple-choice olfactometers are affected in interpretable ways by effects of background colors and airflows. PLoS ONE, 15(7), e0235630, available: https://doi.org/10.1371/journal.pone.0235630
Wiltz, B. and Suiter D. (2007). Deterrence and toxicity of essential oils to Argentine and red imported fire ants (Hymenoptera: Formicidae). Journal of Entomological Science, 42(2), 239-249, available: https://doi.org/10.18474/0749-8004-42.2.239
Zjacic, N. and Scholz, M. (2022). The role of food odor in invertebrate foraging. Genes, Brain and Behavior,21(2), available: https://doi.org/10.1111/gbb.12793