Chloe McCreery1, Izabella Zamora2,3
1 Department of Biological Engineering, Massachusetts Institute of Technology
21 Ames Street #56-651, Cambridge, MA 02142
2 Department of Biology, Massachusetts Institute of Technology
31 Ames Street, Cambridge, MA 02142
3 Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology
50 Vassar Street, Cambridge, MA 02142
Abstract
Monocytes and macrophages are two developmentally related immune cell types that can infiltrate tumors of cancer patients. These cells critically impact cancer progression due to their abilities to both induce and suppress the body’s natural anti-cancer immune response. Since these cell types can directly hinder the efficacy of immunotherapy treatments, identifying strategies to inactivate and inhibit their functions is of great therapeutic interest. In this review, we discuss how monocyte and macrophage populations contribute to the cancer immunity cycle, a cycle which specifically targets cancer cells while keeping healthy cells unharmed. Specifically, our review focuses on the roles of these cells in the blood (circulating monocytes), tumor tissue (tumor-resident macrophages and monocyte-derived dendritic cells), as well as lymph nodes (lymph node-resident macrophages). We discuss how these cells can promote cancer growth and can participate in the immune attack against cancer through the secretion of cytokines, thereby aiding or harming the immune response. We highlight how certain migrating macrophage populations can take up tumor antigens and travel to the lymph node to activate T cells to begin the killing of tumor cells—a role that is usually thought to be fulfilled by only dendritic cells. Lastly, this review highlights why monocytes and macrophages are promising targets for treating cancer, and how these cells can be reprogrammed to improve patient responses to existing therapies, termed immunotherapies, that act to enhance the body’s natural anti-cancer defenses.
Introduction
Under ideal conditions, the body’s immune system is capable of identifying and destroying cancer cells. This process is coined as the cancer immunity cycle, and its success heavily relies on the cytotoxic T cell (Chen and Mellman, 2013). This cell can travel to the environment around the tumor, also called the tumor microenvironment (TME), and use its T cell receptors (TCRs) to recognize specific molecular identifiers (antigens) on tumor cell membranes. Upon recognition, the cytotoxic T cell will kill the tumor cell using cytotoxic enzymes (Chen and Mellman, 2013). However, this process can be hindered by molecules on the T cell membrane that bind to ligands on other cells – referred to as an immune checkpoint(s). This binding consequently inhibits T cell functions (Dyck and Mills, 2017). When cells within the tumor express high levels of inhibitory proteins that interact with T cell checkpoint molecules, they can result in T cell “exhaustion” and prevent further anti-tumor immune responses (Zappasodi et al., 2018).
Immunotherapy treatments that work to prevent this interaction between T cells and tumor cells have been a breakthrough in cancer therapy. Termed checkpoint blockade therapy, this immunotherapy reinvigorates T cells to continue killing cancer, and can induce durable survival benefits in patients with various cancer types (Pardoll, 2012; Pico de Coaña et al., 2015). For example, a checkpoint blockade therapy for mesothelioma was shown in a phase 3 clinical trial to extend the survival of patients who did not respond to chemotherapy (Fennell et al., 2021). This revolutionary method for cancer treatment was awarded the 2018 Nobel Prize in Physiology or Medicine, acknowledging its importance in the realm of oncology (Guo, 2018).
However, even with cancer immunotherapy’s high potential to induce cancer remission for patients who respond to the therapy, the fraction of responsive patients remains low (Sambi et al., 2019). There is a need for an improved understanding of alternative mechanisms that might prevent T cells from effectively killing cancer cells. Certain immune cells have been shown to interfere with immunotherapy, and this review will specifically focus on the role of two of those cell types: monocytes and macrophages.
Monocytes are bone marrow-derived cells that circulate within the bloodstream. They can migrate to a site of inflammation, then differentiate into macrophages or dendritic cells (DCs) to promote an immune response. Monocytes can also secrete cytokines — molecules used to communicate with other cells — thereby affecting immune cells and their functions within the TME (Kratofil et al., 2016).
Macrophages are resident within tissues in both steady and inflamed states. While they can differentiate from monocytes within an inflammatory setting, most of the macrophages in the body develop from early immune cell progenitors during embryo development (Mosser and Edwards, 2009; Epelman et al., 2014). Macrophages play important roles in disease due to their abilities to engulf, digest, and present debris on their membrane. Like monocytes, macrophages also produce cytokines (Geissmann et al., 2014).
In this review, we discuss the cancer immunity cycle, the specific roles monocytes and macrophages have during the cancer immunity cycle, how they interact with immune cells, and how they can aid or prevent tumorigenesis (tumor acquisition of malignant properties). We also discuss current immune checkpoint blockade treatments, and how the functions of monocytes and macrophages can impact the outcome of these therapies. Both monocytes and macrophages influence T cell function, therefore it is important to understand how these cell types interact with each other. Moreover, understanding the complex interplay between these immune cell types has the potential to inspire new combination strategies in cancer treatment, as supplementing T cell treatments with those that target monocytes or macrophages could lead to more effective anti-tumor responses and better prognoses for cancer patients.
The Cancer Immunity Cycle
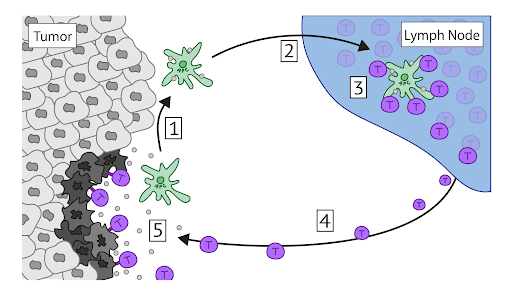
The cancer immunity cycle shown in Figure 1 illustrates the current understanding of the requirements needed to elicit an effective anti-tumor immune response (Richards et al., 2013). It consists of several steps and takes place in multiple locations of the body—including nearby lymph nodes, blood and lymph vessels—in addition to the TME itself.
The first step of this cycle involves antigen-presenting cells (APCs) within the TME. APCs are a subset of immune cells that are capable of taking up cellular debris. An APC captures cellular debris from dying tumor cells, processes it into smaller peptide fragments, then presents those peptides on the APC’s cell surface using major histocompatibility complex (MHC) molecules located on their cell membrane. This process is referred to as cross-presentation (Embgenbroich and Burgdorf, 2018). Dendritic cells (DCs), macrophages, monocytes and B cells are all considered APCs (Chen and Mellman, 2013).
During the process of cross-presentation, the APC matures and activates — this is because dying tumor cells contain pathogen-associated molecular patterns (PAMPs), as well as damage-associated molecular patterns (DAMPs). These patterns are recognized by pattern-recognition receptors (PRRs) within APCs, and this recognition alerts the APC to activate (Zelenay and Reis e Sousa, 2013). Upon activation, the APC travels through lymphatic vessels into the lymph node to elicit a T cell response (Martin-Fontecha et al., 2013). For examples of common PAMPs and DAMPs, refer to Hernandez et al., 2016 and Zelenay and Reis e Sousa, 2013.
Once the APC is within the lymph node, it activates tumor-reactive T cells, which have receptors specific to the APC’s presented tumor antigen. This is done via cross-priming, the process in which an APC presents an antigen on an MHC molecule to a T cell. Cross-priming and activation lead T cells to proliferate and acquire cytotoxic functions (Gutiérrez-Martínez et al., 2015). Cytotoxic T cells will then enter the bloodstream to migrate to and infiltrate the tumor. Once inside the tumor, the T cells recognize and bind to cancer cells then release cytotoxic molecules, which will subsequently kill the cancer cells and eradicate the tumor. T-cell mediated killing causes the cancer immunity cycle to repeat, as it results in more dying tumor cells releasing antigens into the TME for APCs to recognize (Chen and Mellman, 2013).
Figure 1. Cancer Immunity Cycle. The cancer immunity cycle begins within the tumor microenvironment (TME), where an antigen presenting cell (APC) (green) takes up antigen from dying tumor cells (gray) (1). The APC then travels to the lymph node (2), where it activates antigen-specific T cells (purple) (3). These T cells then migrate back to the tumor site (4) where they bind to and kill tumor cells using cytotoxic enzymes (5). This cell-mediated killing gives rise to more tumor antigens that are then released into the environment, allowing the cycle to continue.
Monocytes and Macrophages Can Directly Facilitate Tumor Progression
It may seem counterintuitive for a tumor to attract immune cells. However, for cancer cells to grow and divide without detection, they recruit and take advantage of myeloid cells to induce their anti-inflammatory, pro-tumorigenic effects — this leaves the tumor “shielded” from other immune cells. One such cell type is the monocyte, a bone marrow-derived cell that can be classified as classical or alternative (Figure 2). Classical monocytes act as first responders during an immune response. They enter the diseased or damaged tissue site and induce further inflammation via the recruitment of various immune cells. Classical monocytes typically differentiate into monocyte-derived DCs (moDCs) capable of migrating to nearby lymph nodes to promote inflammation (Shi, 2011).
Besides the classical monocyte, there is the alternative monocyte, which tends to patrol tissues or reside within them and can be found rolling along the surface of blood vessels in non-inflamed tissues. As a consequence, the alternative monocyte cells are also among the first to infiltrate an infected site. However, unlike the classical subset, alternative monocytes typically differentiate into macrophages (Auffray et al., 2007). In cancer, monocytes are generally recruited via the molecule CCL2. Once in the TME, monocytes can differentiate into either moDCs or macrophages.
Macrophages in the TME are called tumor-associated macrophages or TAMs (Qian et al., 2011). The origin of TAMs is not well studied; however, some research suggests the majority of TAMs are derived from circulating monocytes that are recruited into the tumor (Olingy et al., 2019). These tumor-resident macrophages can make up approximately 50% of a tumor’s mass (Vinogradov et al., 2014). TAMs can be activated to become one of two general states: proinflammatory or anti-inflammatory. Proinflammatory classically activated macrophages are activated by the cytokine interferon gamma (IFNγ), while anti-inflammatory alternatively activated macrophages are induced through exposure to cytokines interleukin 4 (IL-4) and 13 (IL-13) (Murray et al., 2014) (Figure 2). IL-4 and IL-13 have similar functions, as they both inhibit proinflammatory pathways (Yokota et al., 1986; Junttila, 2018). Furthermore, the engulfment of tumor debris can cause macrophages to become alternatively activated through the STAT6 signaling pathway, thereby preventing tumor detection and suppressing responses from other immune cells due to its anti-inflammatory properties (Ma et al., 2016; Pio et al., 2019).
Figure 2. Monocytes and macrophages generally divide into two groups: classical and alternative. Classical monocytes are among the first immune cells to respond to disease or damage in tissues and can differentiate into monocyte-derived Dendritic Cells (moDCs) and tissue-resident macrophages. On the other hand, alternative monocytes are more likely to differentiate into tissue-resident macrophages and patrol non-inflamed tissues. In the case of macrophages, classical macrophages are generally activated via IFNG and exhibit a pro-inflammatory phenotype. Alternative macrophages can be activated through IL-4 and IL-13, or through the STAT-6 signaling pathway, and exhibit an anti-inflammatory phenotype, secreting molecules such as TGFβ and PGE2. Both macrophage types can contribute up to 50% of the mass of a tumor.
In addition to preventing tumor detection, TAMs can also aid in tumor spread to other tissues (metastasis), blood vessel growth into the tumor (angiogenesis), and invasion. TAMs have been shown to upregulate pathways that can enhance cancer cells’ migratory and invasive abilities in human colorectal cancer (Wei et al., 2019), facilitating a process called the epithelial-to-mesenchymal (EMT) transition, which aids metastasis. Furthermore, TAMs can secrete vascular endothelial growth factor A (VEGF-A), a growth factor which induces tumor progression through angiogenesis in the context of breast cancer (Lin et al., 2007). Along with VEGF-A, TAMs also secrete metalloproteases, or MMPs, that degrade the extracellular matrix allowing tumors to have more room to expand and grow further (Coussens et al., 2002).
Both monocytes and macrophages secrete diverse cytokines into the TME, which can directly facilitate tumor growth (Richards et al. 2013; Qian et al., 2011). For example, alternatively activated TAMs can produce transforming growth factor beta (TGFβ) and prostaglandin E2 (PGE2) (Noy and Pollard, 2014). Both TGFβ and PGE2 are immunosuppressive, anti-inflammatory molecules capable of promoting cancer malignancy (Ke et al., 2016; Seoane and Gomis, 2017). Monocytes can also inhibit an immune response by migrating to the tumor via the CCR5/CCL5 axis and producing IL-6 in the TME (Anand et al., 1998; Aldinucci et al., 2020). Most of the time, IL-6 acts to promote cancer cell proliferation and metastasis, aiding tumor evasion and angiogenesis. However, IL-6 also has the capacity to increase T cell trafficking to the lymph node. This duality shows the potential for IL-6 to be beneficial to the tumor microenvironment and potentially a therapeutic target (Fisher et al., 2014). The CCR5/CCL5 axis similarly has mixed effects in cancer, having been associated with tumor progression and metastasis in addition to aiding anti-tumor responses. This happens by the axis inducing the activation and proliferation of natural killer cells, and recruiting T cells along with other immune cells (Aldinucci and Colombatti 2014).
Monocytes and Macrophages Affect Tumor antigen presentation by APCs
For the cancer immunity cycle to begin, APCs must be able to present and acquire tumor antigens. This is vital as these tumor antigens are necessary when APCs later travel to the lymph node and activate T cells with those antigens. In the case of cancer, the APCs that initiate this cancer immunity cycle are usually DCs. However, cytokines produced by monocytes and macrophages in the TME, in addition to the tumor itself, can inhibit DC differentiation, maturation, activation, and consequently, their function — this means cancer immunity cycle cannot begin.
With regards to DC differentiation, tumor cells can produce IL-6 and lactic acid, which can inhibit the differentiation of monocytes into DCs and can suppress DC maturation and survival (Gottfried et al., 2006; Menetrier-Caux et al., 1998). Another important molecule which affects DC function is IL-10, which halts DC maturation and antigen presentation. Monocytes, macrophages and moDCs can release this cytokine (Zong et al., 2016), and it inhibits the ability of DCs to create antigen presentation molecules, thereby reducing the ability to activate T cells (Williams et al., 2004). IL-10 has also been shown to interfere with the maturation of DC populations by inhibiting the expression of the costimulatory molecule CD40, which is needed for optimal T cell activation in colon cancer along with costimulatory molecules CD80 and CD86 (Shurin et al., 2002).
Macrophages have also been shown to have a suppressive effect on DCs. In particular, TGFβ, produced by TAMs, can inhibit DCs (Batlle and Massagué, 2019). Multiple studies highlight the function of TGFβ as a pro-tumorigenic molecule: for example, in bile duct cancer, also known as cholangiocarcinoma, inhibition of TGFβ receptors on DCs allowed for them to interact less with TGFβ in the TME and caused these DCs to activate T cells more effectively in the lymph node (Thepmalee et al., 2018). In another study, blocking TGFβ receptors aided DCs ability to inhibit tumor growth in a breast cancer model in vivo (Kobie et al., 2003). A similar effect was shown in colon cancer wherein DCs that were exposed to TMEs expressing TGFβ failed to produce effective T cell responses (Kao et al., 2003).
In contrast to the potential inhibitory effects of macrophages, these cells may also be capable of initiating the cancer immunity cycle as an APC. An example of such are Langerhans cells, a subset of macrophages that mostly reside within the epidermis layer of the skin. It has been observed in vivo that Langerhans cells are capable of traveling from the skin to the lymph node in order to present tumor antigens (Cohen et al., 1994).
There is also some evidence showing macrophages phagocytosing and cross-presenting cellular debris in vitro (Muntjewerff et al., 2019). In irradiated melanoma cells, human macrophages were able to activate cytotoxic T cells in vitro (Barrio et al., 2012). In addition, bone marrow derived macrophages can even present antigens in the presence of inhibitory molecules that prevent cross-presentation in DCs (Cruz-Leal et al., 2018). However, although macrophages are capable of cross-presentation, DCs remain superior in this function. For example, DCs specialized in cross-presentation have been shown to have their presented peptides degrade at a slower rate, increasing their presentation ability (Savina et al., 2006). Although their research suggests that macrophages may have limited ability to replace or complement DC functions in the cancer immunity cycle, more research is needed to understand to what degree cross-presentation in macrophages can contribute to cancer control.
Monocytes and Macrophages in the Lymph Node
If a DC successfully takes up a tumor antigen and becomes activated, it will then travel to the lymph node to activate tumor-reactive T cells — macrophages and monocytes also reside here and can influence the anti-tumor response. It remains unclear whether macrophages can also migrate from tumors into the lymph node; however, limited reports in non-cancer contexts have suggested that macrophage migration is possible. Infiltrating macrophages in the epidermal and dermal layers of the skin of mice have been observed to migrate to a draining lymph node upon exposure to UV irradiated skin cells (Toichi et al., 2008). In addition, murine alveolar macrophages have been shown to migrate from lung alveoli to the lung draining lymph node after being exposed to gram-positive bacteria (Kirby et al., 2009). Thus, limited evidence suggests that macrophages are capable of migrating from tissue to the draining lymph node, but more research is needed to understand whether this can occur in the tumor setting as well.
There is also limited evidence to suggest that monocytes and moDCs may be able to migrate to the lymph node and activate T cells. For example, murine inflammatory monocytes, marked by their high expression of the protein LY6C, have been considered capable of presenting antigens collected from a site of inflammation in the context of infection and activating cytotoxic T cells both in vitro and in vivo (Gautier et al., 2012; Leirião et al., 2012). This may suggest their capacity to enhance anti-tumor functions in the cancer context. MoDCs are also able to migrate to the lymph node to activate T cells, just like any other DC (Wculek et al., 2019). MoDCs can induce different types of CD4+ helper T cell responses, including the type I response, needed for enhancing cytotoxic T cell activation and their anti-tumor responses (Kaiko et al., 2008; Bellik et al., 2006).
Aside from performing the role of APCs, monocytes and macrophages can influence the cancer immunity cycle in the lymph node in other ways. One group of macrophages residing in the lymph node are subcapsular sinus (SCS) macrophages, which line and encompass the entire organ (Gray and Cyster, 2012). These macrophages are often located at the entrance of the lymph node where they can filter debris from blood and lymphatic fluid (Barral et al., 2010). SCS macrophages are characterized by their expression of CD169, a cell adhesion molecule that promotes hematopoietic stem cell retention (Louie and Liao, 2019). In a healthy context, SCS macrophages serve to capture pathogens within the lymph node, activating T cells in response to both bacterial and viral infections (Junt et al., 2007; Moran et al., 2019).
Interestingly, the presence of SCS macrophages has been shown to correlate with better prognoses among multiple cancer models. For example, in melanoma, SCS macrophages were shown to suppress tumor growth by blocking the immune-inhibitory effects of tumor-derived extracellular vesicles (Pucci et al., 2016). These extracellular vesicles are created by tumor cells and can carry tumor antigens throughout the body through the lymphatic system. However, SCS macrophages can physically block these vesicles from interacting with other immune cells within the lymph node, thereby blocking tumor communication and preventing tumor progression (Pucci et al., 2016).
High numbers of SCS macrophages in lymph nodes are associated with increased tumor infiltration of cytotoxic T cells across esophageal, breast and bladder cancers. In all three cancer types, high expression of CD169 on SCS macrophages is linked to tumor-infiltrating lymphocytes and a higher potential for anti-cancer immunity (Takeya et al., 2018; Shiota et al., 2016; Asano et al., 2018). CD169 expression is also correlated with proinflammatory macrophage polarization in esophageal cancer (Takeya et al., 2018), lower levels of tumor cell proliferation and metastasis in breast cancer and a higher 5-year survival rate in patients with bladder cancer (Shiota et al., 2016; Asano et al., 2018). Thus, these SCS macrophages appear to be capable of facilitating anti-tumor responses within multiple cancer models. Further research might reveal therapeutic strategies for tuning SCS macrophage function to improve tumor control.
Both macrophages and monocytes in the lymph node play a role in T cell activation. SCS macrophages have been shown to activate T cells to a similar extent like DCs in vivo during a lymphocytic choriomeningitis virus (LCMV) infection, which primarily infects DCs. However, in order to activate the same amount of T cells as a DC, SCS macrophages in this study required significantly more peptides, making these macrophages inferior APCs (Pozzi et al., 2005). Despite this, CD169,+SCS macrophages and DCs together seem to be able to activate T cells recognizing a broader range of tumor antigens compared to DCs alone (Bernhard et al., 2014).
As for monocytes, they have been known to promote proper T cell activation and function through the secretion of IL-15 and IL-18, both proinflammatory molecules. Inflammatory monocytes are the main producer of these stimulatory cytokines, suggesting that therapies to boost their production may be useful in mounting an anti-tumor response. (Soudja et al., 2012). Furthermore, IL-15 can induce differentiation of monocytes into moDCs in vitro (Saikh et al., 2001; Mohamadzadeh et al., 2001). MoDCs can also promote T cells to become type 2 helper T cells, a group associated with tumor progression and poor prognosis (Mantovani et al. 2008; Wculek et al., 2020). However, there is evidence suggesting that these moDCs are short-lived and dysfunctional (Onishi et al., 2002; Kiertscher et al., 2000).
Recruitment of tumor-reactive T cells and their cytotoxic functions
Once T cells are primed and activated in the lymph node, they travel to the tumor site and infiltrate the tumor. Within the TME, T cells can interact with MoDCs and macrophages, and depending on the context, these interactions can be inhibitory or stimulatory. Inhibitory effects of moDCs and macrophages can result from their expression of the checkpoint blockade molecule PDL1, which can directly inhibit T cells by interacting with the PD1 receptor (Bakdash et al., 2016; Spary et al., 2014). In this case, PDL1 blocking treatment has the potential of rescuing T cells from such inhibitory interactions. Similarly, blocking treatment against CTLA-4, another checkpoint molecule, has been shown to decrease the number of inhibitory macrophages while promoting T cell activation, thereby enabling the migration of the T cells out of the lymph node in vivo (Yu et al., 2016).
MoDCs can also promote anti-tumor immune responses by producing the stimulatory IL-12 cytokine needed for activating T cells. However, compared to type 1 conventional DCs, the contribution of moDCs to IL-12 production appears to be small (León et al., 2007). The role of moDC in anti-tumor immune responses is still an active area of investigation and is complicated by the difficulty of distinguishing moDC from other DC populations in the tumor. Further research is necessary to better understand how these cells can influence tumor control.
In addition to their role in inhibiting T cell activation, tumor macrophages are also associated with reducing the ability of T cells to migrate into the tumor. Contact between T cells and macrophages in lung squamous cell carcinoma tumors has been observed to confine T cells to the outer regions of the tumor, thereby preventing further infiltration (Peranzoni et al., 2018). Thus, the depletion of macrophages from the TME could result in increased T cell migration and activity (Peranzoni et al., 2018).
If T cells successfully infiltrate the tumor, macrophages may still suppress their ability to recognize and kill tumor cells. This is because TAMs secrete a cytokine referred to as hypoxia inducible factor alpha (HIF-1α). In addition to its ability to promote tumor growth, HIF-1α can suppress T cells in a hypoxic environment (Doedens et al., 2010). TAMs have also been suggested to kill activated T cells altogether and reduce proliferation. In vitro, macrophages that produce tumor necrosis factor (TNF) and related receptor genes secrete nitric oxide and arginase which can kill T cells (Saio et al., 2001). The STAT1 pathway, which leads to the transcription of interferon-stimulated genes and correlates with tumor growth, may also be involved with this TAM-mediated cell death (Khodarev et al., 2012). STAT1+/+TAMs were able to severely reduce T cell proliferation, while STAT1-/-TAMs were unable to do the same (Khodarev et al., 2012). This finding highlights the importance of STAT1 to hinder the cancer immunity cycle’s tumor-killing abilities.
Current Immunotherapies Targeting Macrophages and Monocytes
Checkpoint blockade therapy targets immune cell receptors or ligands to block immunosuppressive cell signaling and encourage an anti-tumor immune response (Figure 3). This form of therapy can function at the tumor site, blocking signals on tumor cells that would otherwise prevent killing, or in the lymph node, blocking inhibitory signals between APCs and T cells thereby leading to the better activation of T cells. Two of the more well-known checkpoint blockade therapies include anti-PD1/PDL1 therapy and anti-CTLA-4 therapy.
Anti-PD1/PDL1 therapy acts by inhibiting the interaction between PD1 and PDL1. This is an effective therapy because PD1 is an inhibitory molecule for T cells, and the connection of PD1 and PDL1 creates a “shield” for tumor cells to remain undetected by the immune system. Preventing this interaction via anti-PD1/PDL1 therapy allows cytotoxic T cells to remain activated, allowing them to kill tumor cells (Marchetti et al., 2017).
Anti-CTLA-4 therapy targets the cytotoxic T lymphocyte antigen 4 (CTLA-4). This protein is a receptor on the T cell membrane and is upregulated once the T cells become exhausted (Arlauckas et al., 2017). Upon CTLA-4 binding to CD80 or 86, T cell functions are inhibited, and they are no longer able to kill cancer cells. Similar to anti-PD1 therapy, anti-CTLA-4 therapy works to prevent its respective proteins, CTLA-4 and CD80/86, from binding in order to maintain T cell mediated killing of tumor cells (Seidel et al., 2018) (Figure 3). Macrophages and monocytes are both associated with this form of immunotherapy, as they can express CD80/86 and interact with CTLA-4 proteins on T cells.
Figure 3. PD1 and CTLA-4 Checkpoint Blockade Therapy. The effect of PD-1 binding to PD-L1, as well as CTLA-4 to CD80/86, on the anti-tumor response can severely limit a T cell’s capacity to kill cancer cells. With checkpoint blockade therapy (anti-PD-1 and anti-CTLA-4 administration) to block these interactions, immunosuppressive signaling is prevented, allowing for the activation and migration of T cells, and therefore tumor clearance. In the case of anti-CTLA-4 therapy, the interaction between CTLA-4 on a T cell and CD80/86 on an antigen presenting cell (APC) in the lymph node is blocked, so co-stimulatory signaling can occur, leading to T cell activation and migration to the tumor site. For anti-PD-1 therapy, the PD-1 ligand on the T cell is blocked to prevent binding to a tumor cell’s PD-L1 ligand, then the T cell can successfully kill tumor cells.
To combat this problem, CTLA4Ig, a soluble form of the CTLA-4, has been used to block CD80/86 on macrophages and their interactions with DCs, B cells, and T cells (Gao et al., 1999; Lane et al., 1993). CTLA4Ig is a fusion protein consisting of the extracellular region of a mouse CTLA4 gene and a human IgG1 antibody constant. CTLA4Ig binds to B cells, DCs and T cells, and upon binding, the ability of antigen presenting cells to properly create clones of antigen specific T cells is inhibited (Lane et al., 1993). Today, CTLA4Ig is FDA approved to treat rheumatoid arthritis preventing self-tolerance for autoreactive T cells (Bluestone, 2006).
Today, immune checkpoint blockades used in therapy include blocking agents for the molecules PD1, PDL1, and CTLA-4. The PD1 blocking antibody Pembrolizumab (Keytruda) was approved starting in 2014 for advanced melanoma that was untreatable by traditional methods, and in subsequent years, it was adopted for additional types of cancer, particularly in patients with PD1 expressing tumors (Emancipator, 2021). Since 2014, other PD1 blocking therapies have come to market and have shown various efficacy across different treatment conditions. The differences molecularly and experimentally are being explored today to better inform clinical decisions. For more information, refer to Chen et al., 2020. Similarly, the anti-PDL1 blocking antibody Atezolizumab (Tecentriq) was approved in 2016 for non-small cell lung cancer. Other PDL1 drugs approved include Durvalumab and Avelumab, which were both approved in 2017 (Neumann et al.,, 2022). In 2011, the first CTLA-4 inhibitor, Ipilimumab, was FDA approved and is used to treat many different cancer types. It has proven to be most effective in combination with PD1 or PDL1 blocking therapies (Rotte, 2019).
These types of checkpoint blockade therapies have been shown to be hindered by the presence of TAMs – for example, depleting TAMs with the drug Pexidartinib (PLX3397) and treating mice with anti-PD1 therapy was shown to be more effective in reducing tumor size and recruiting T cells than anti-PD1 therapy alone, suggesting that TAMs can reduce anti-PD1 therapy efficacy (Peranzoni et al., 2018). It has also been shown that macrophages can inactivate T cells via removing anti-PD1 antibodies on T cells, thus ablating the ability of these antibodies to rescue cytotoxic T cell function within the tumor (Peranzoni et al., 2018). Thus, ablating this population of antibodies in conjunction with checkpoint blockade therapy could prove useful in promoting successful responses to the therapy – however, more research still needs to be done in this area to test these findings clinically.
There has been research into targeting monocytes and preventing their accumulation in the TME through the use of small interfering RNA (siRNA), which binds to messenger RNA and interferes with translation of that messenger RNA into a protein. These siRNA are targeted towards the protein CCR2, which attracts monocytes (Leuschner et al., 2011). On the other hand, moDCs have been targeted through the blocking of their inhibitory, pro-tumorigenic functions in favor of their stimulatory, anti-tumorigenic functions. Examples of such targeting are DC vaccines — these vaccines consist of moDCs differentiated from monocytes that have been taken from a patient. The moDCs are cultured ex vivo, exposed to the tumor antigen, and injected back into the patient in order to stimulate an immune response. Unfortunately, these vaccines have not been shown to induce strong therapeutic responses – this is largely due to the fact that moDCs do not have the full capabilities of a regular DC (Calmeiro et al., 2020). Another therapeutic strategy includes targeting receptor molecules on moDCs to inhibit moDC immunosuppressive activity (Birkholz et al., 2010; Tacken et al., 2005; Hutten et al., 2016; Yin et al., 2016).
Besides checkpoint blockade therapy, there is also evidence of macrophages playing a role in immunotherapies involving tumor-targeting antibodies. Under this branch of immunotherapy, antibodies bind to specific proteins on the surface of tumor cells. An example of this is trastuzumab, an antibody that can bind to the HER2 receptor, which is overexpressed in certain breast cancers. Upon binding, trastuzumab inhibits proliferation of the cell it is bound to, and, as a result, it can prevent cancer growth. Tumor-targeting antibody immunotherapies like trastuzumab can also aid in cancer cell phagocytosis, partly due to their interactions with macrophages. This is because macrophages are able to recognize and phagocytose tumor cells bound to the antibody in a phenomenon known as antibody-dependent cellular phagocytosis (Shi et al., 2015). Once again, we are reminded of the enormous potential macrophages have in enhancing current and future therapeutic endeavors in cancer.
Discussion
In this review, we have described how monocytes and macrophages are involved in regulating tumor development. In addition to directly affecting tumor cell growth, these immune cells can positively and negatively regulate anti-tumor immune responses at several stages of the cancer immunity cycle, including tumor antigen presentation, antigen transport to the lymph node and subsequent T cell activation and recruitment into the tumor. This is usually achieved through the expression of cytokines and growth factors (see Table 1). Consequently, monocytes and macrophages can and do majorly influence the efficacy of immunotherapies in use today.
Table 1. Cytokines and Growth Factors involved in the Cancer Immunity Cycle
Pro-inflammatory cytokines | Function | Expression |
CCL2 | Recruits monocytes to a site of inflammation. | Dendritic cells, monocytes, macrophages |
CSF-1 | Promotes proliferation and differentiation of monocytes and macrophages. | Macrophages |
IFNI | Activates T cells and NK cells. | CD169+ macrophages |
IFNγ | Activates macrophages; induces MHC II molecule production. | T cells, natural killer cells |
IL-1 | Activates T cells and NK cells. | CD169+ macrophages |
IL-12 | Promotes T cell anti-tumor responses. | cDC1s, monocyte-derived dendritic cells |
IL-15 | Vital for proper T cell activation and function. | Monocytes |
IL-18 | Activates T cells and NK cells. | Monocytes, CD169+ macrophages |
IL-33 | Activates APCs. | Tumor cells |
Anti-inflammatory cytokines | Function | Expression |
CCL5 | Attracts CCR5+ monocytes to the tumor site. | Tumor cells |
HIF-1A | Promotes tumor growth; suppresses T cell functions. | Macrophages |
IL-4 | Promotes alternative activation in macrophages. | CD4+ T cells |
IL-6 | Promotes tumor progression; inhibits monocyte differentiation and DC survival. | Monocytes, macrophages |
IL-10 | Blocks T cell costimulatory molecules (CD28, CD40, CD80, CD86); inhibits DC function maturation and presentation. | Dendritic cells, monocytes, macrophages |
IL-13 | Promotes alternative activation in macrophages. | CD4+ T cells |
PGE2 | Anti-inflammatory molecule. | Macrophages |
TGFβ | Anti-inflammatory molecule; suppresses DC functions. | Macrophages |
Growth Factors | Function | Expression |
MMPs | Degrades extracellular matrix proteins. This gives tumors more room to grow. | Macrophages |
VEGF-A | Promotes angiogenesis within the TME. | Macrophages |
Since the TME can polarize monocytes and macrophages to acquire pro-tumorigenic properties, these cells largely act to support tumor growth. This is done through the secretion of molecules that suppress immune responses as well as promote growth, metastasis, and blood vessel growth. As a result, cancer cells become protected from much-needed anti-tumor responses and gain nutrients to grow, divide, and potentially spread to other parts of the body. Current immunotherapies blocking these functions include checkpoint blockade therapies such as anti-PD1/PDL1 and anti-CTLA-4 therapy. These therapies block interactions that make tumors effectively “invisible” to the immune system, thereby promoting an immune response on cancer cells. These therapies have also been used across a wide variety of cancers (FDA, 2022).
Although the effects monocytes and macrophages have on tumor progression are predominantly negative, depending on the context, it is possible for monocytes and macrophages to execute more proinflammatory and anti-tumorigenic functions. These cell types have the potential to present tumor antigens and activate T cells, thus supporting the cancer immunity cycle. As such, it is clear that polarizing monocytes and macrophages to mount an anti-tumor response has the potential to promote T cell-mediated killing of cancer cells. Research is still limited regarding co-targeting monocytes and macrophages alongside checkpoint blockade in humans – however, studies involving checkpoint blockade in conjunction with TAM ablation in animal models have shown promising results in reducing tumor size and improving T cell recruitment. This points to a growing need for research into applying TAM-targeting from the lab to the clinic.
Overall, understanding how monocytes and macrophages regulate immune responses against tumors and how we could promote the anti-tumor functions of these cells is vital for creating and/or enhancing current immunotherapies to combat tumors and improve patient outcomes. Ultimately, pairing therapies that target monocytes and macrophages with checkpoint blockade immunotherapy is a promising therapeutic strategy, which could improve response rates and thus extend the benefits of immunotherapy to a larger number of patients.
Acknowledgements
We would like to express thanks to the Spranger Lab for providing us the opportunity to learn about tumor immunology, and to be a part of the team. We would especially like to thank Maria Zagorulya for providing expertise, guidance, and continuous support as our direct supervisor. We also appreciate the generous funding and support received from the Undergraduate Research Opportunities Program at the Massachusetts Institute of Technology.
References
Aldinucci, D. and Colombatti, A. (2014) ‘The inflammatory chemokine CCL5 and cancer progression’, Mediators of inflammation, 2014 available: https://doi.org/10.1155/2014/292376
Aldinucci, D., Borghese, C. and Casagrande, N. (2020) ‘The CCL5/CCR5 Axis in Cancer Progression’, Cancers, 12(7), available: https://doi.org/10.3390/cancers12071765
Anand, M., Chodda, S.K., Parikh, P.M. and Nadkarni, J.S. (1998) ‘Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis’, Hematological. Oncology, 16, 143-154, available: https://doi.org/10.1002/(SICI)1099-1069(199812)16:4<143::AID-HON628>3.0.CO;2-U
Arlauckas, S. P., Garris, C. S., Kohler, R. H., Kitaoka, M., Cuccarese, M. F., Yang, K. S., Miller, M. A., Carlson, J. C., Freeman, G. J., Anthony, R. M., Weissleder, R. and Pittet, M. J. (2017) ‘In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy’, Science Translational Medicine, 9(389), available: https://doi.org/10.1126/scitranslmed.aal3604
Asano, T., Ohnishi, K., Shiota, T., Motoshima, T., Sugiyama, Y., Yatsuda, J., Kamba, T., Ishizaka, K. and Komohara, Y. (2018) ‘CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis’, Cancer Science, 109(5), 1723–1730, available: https://doi.org/10.1111/cas.13565
Auffray, C., Fogg, D., Garfa, M., Elain, G., Join-Lambert, O., Kayal, S., Sarnacki, S., Cumano, A., Lauvau, G. and Geissmann, F. (2007) ‘Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior’, Science, 317(5838), 666–670, available: https://doi.org/10.1126/science.1142883
Bakdash, G., Buschow, S. I., Gorris, M. A. J., Halilovic, A., Hato, S. V., Sköld, A. E., Schreibelt, G., Sittig, S. P., Torensma, R., Duiveman-de Boer, T., Schröder, C., Smits, E. L., Figdor, C. G. and de Vries, I. J. M. (2016) ‘Expansion of a BDCA1+CD14+ Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines’, Cancer Research, 76(15), 4332–4346, available: https://doi.org/10.1158/0008-5472.CAN-15-1695
Balkwill, F. R., Capasso, M. and Hagemann, T. (2012) ‘The tumor microenvironment at a glance’, Journal of Cell Science, 125(23), 5591–5596, available: https://doi.org/10.1242/jcs.116392
Barral, P., Polzella, P., Bruckbauer, A., van Rooijen, N., Besra, G. S., Cerundolo, V. and Batista, F. D. (2010) ‘CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes’, Nature Immunology, 11(4), 303–312, available: https://doi.org/10.1038/ni.1853
Barrio, M. M., Abes, R., Colombo, M., Pizzurro, G., Boix, C., Roberti, M. P., Gélizé, E., Rodriguez-Zubieta, M., Mordoh, J. and Teillaud, J.-L. (2012) ‘Human macrophages and dendritic cells can equally present MART-1 antigen to CD8(+) T cells after phagocytosis of gamma-irradiated melanoma cells’ PloS One, 7(7), e40311, available: https://doi.org/10.1371/journal.pone.0040311
Batlle, E. and Massagué, J. (2019) ‘Transforming Growth Factor-β Signaling in Immunity and Cancer’, Immunity, 50(4), 924–940, available: https://doi.org/10.1016/j.immuni.2019.03.024
Bellik, L., Gerlini, G., Parenti, A., Ledda, F., Pimpinelli, N., Neri, B. and Pantalone, D. (2006) ‘Role of conventional treatments on circulating and monocyte-derived dendritic cells in colorectal cancer’, Clinical Immunology , 121(1), 74–80, available: https://doi.org/10.1016/j.clim.2006.06.011
Bernhard, C. A., Ried, C., Kochanek, S. and Brocker, T. (2015) ‘CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells’, Proceedings of the National Academy of Sciences of the United States of America, 112(17), 5461–5466, available: https://doi.org/10.1073/pnas.1423356112
Binnewies, M., Mujal, A. M., Pollack, J. L., Combes, A. J., Hardison, E. A., Barry, K. C., Tsui, J., Ruhland, M. K., Kersten, K., Abushawish, M. A., Spasic, M., Giurintano, J. P., Chan, V., Daud, A. I., Ha, P., Ye, C. J., Roberts, E. W. and Krummel, M. F. (2019) ‘Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity’, Cell, 177(3), 556–571.e16, available: https://doi.org/10.1016/j.cell.2019.02.005
Birkholz, K., Schwenkert, M., Kellner, C., Gross, S., Fey, G., Schuler-Thurner, B., Schuler, G., Schaft, N. and Dörrie, J. (2010) ‘Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation’, Blood, 116(13), 2277–2285, available: https://doi.org/10.1182/blood-2010-02-268425
Bluestone, J. A., St Clair, E. W. and Turka, L. A. (2006) ‘CTLA4Ig: bridging the basic immunology with clinical application’, Immunity, 24(3), 233–238, available: https://doi.org/10.1016/j.immuni.2006.03.001
Calmeiro, J., Carrascal, M. A., Tavares, A. R., Ferreira, D. A., Gomes, C., Falcão, A., Cruz, M. T. and Neves, B. M. (2020) ‘Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells’, Pharmaceutics, 12(2), available: https://doi.org/10.3390/pharmaceutics12020158
Chen, D. S. and Mellman, I. (2013) ‘Oncology meets immunology: the cancer-immunity cycle’, Immunity, 39(1), 1–10, available: https://doi.org/10.1016/j.immuni.2013.07.012
Chen, Y., Pei, Y., Luo, J., Huang, Z., Yu, J. and Meng, X. (2020) ‘Looking for the Optimal PD-1/PD-L1 Inhibitor in Cancer Treatment: A Comparison in Basic Structure, Function, and Clinical Practice’, Frontiers in immunology, 11, 1088, available: https://doi.org/10.3389/fimmu.2020.01088
Cohen, P. J., Cohen, P. A., Rosenberg, S. A., Katz, S. I. and Mulé, J. J. (1994) ‘Murine epidermal Langerhans cells and splenic dendritic cells present tumor-associated antigens to primed T cells’ European Journal of Immunology, 24(2), 315–319, available: https://doi.org/10.1002/eji.1830240206
Coussens, L. M., Fingleton, B. and Matrisian, L. M. (2002) ‘Matrix metalloproteinase inhibitors and cancer: trials and tribulations’, Science, 295(5564), 2387–2392, available: https://doi.org/10.1126/science.1067100
Cruz-Leal, Y., Grubaugh, D., Nogueira, C. V., Lopetegui-González, I., Del Valle, A., Escalona, F., Laborde, R. J., Alvarez, C., Fernández, L. E., Starnbach, M. N., Higgins, D. E. and Lanio, M. E. (2018) ‘The Vacuolar Pathway in Macrophages Plays a Major Role in Antigen Cross-Presentation Induced by the Pore-Forming Protein Sticholysin II Encapsulated Into Liposomes’, Frontiers in Immunology, 9, 2473, available: https://doi.org/10.3389/fimmu.2018.02473
Doedens, A. L., Stockmann, C., Rubinstein, M. P., Liao, D., Zhang, N., DeNardo, D. G., Coussens, L. M., Karin, M., Goldrath, A. W. and Johnson, R. S. (2010) ‘Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression’, Cancer Research, 70(19), 7465–7475, available: https://doi.org/10.1158/0008-5472.CAN-10-1439
Dyck, L. and Mills, K. (2017) ‘Immune checkpoints and their inhibition in cancer and infectious diseases’, European journal of immunology, 47(5), 765–779, available: https://doi.org/10.1002/eji.201646875
Emancipator, K. (2021) ‘Keytruda and PD-L1: a Real-World Example of Co-development of a Drug with a Predictive Biomarker’, AAPS J, 23(1), 5, available: https://doi.org/10.1208/s12248-020-00525-1
Embgenbroich, M. and Burgdorf, S. (2018) ‘Current Concepts of Antigen Cross-Presentation’, Frontiers in Immunology, 9, 1643, available: https://doi.org/10.3389/fimmu.2018.01643
Epelman, S., Lavine, K. J. and Randolph, G. J. (2014) ‘Origin and functions of tissue macrophages’, Immunity, 41(1), 21–35, available: https://doi.org/10.1016/j.immuni.2014.06.013
Fennell, D. A., Ewings, S., Ottensmeier, C., Califano, R., Hanna, G. G., Hill, K., Danson, S., Steele, N., Nye, M., Johnson, L., Lord, J., Middleton, C., Szlosarek, P., Chan, S., Gaba, A., Darlison, L., Wells-Jordan, P., Richards, C., Poile, C., Lester, J. F., Griffths, G., Price, G., Shaw, P., Cave, J., Naik, J., Ford, A., Geldhart, T., Dancey, G., Papadatos, D., Polychronis, A., Jankowska, P., Scott, A., Gardiner, J., Cominos, M., Campbell, L., MacGregor, C., Mullholand, L., Meenali, C. and Dougherty, G. (2021) ‘Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial’, The Lancet Oncology, 22(11), 1530–1540, available: https://doi.org/10.1016/S1470-2045(21)00471-X
Fisher, D. T., Appenheimer, M. M. and Evans, S. S. (2014) ‘The two faces of IL-6 in the tumor microenvironment’, Seminars in immunology, 26(1), 38–47, available: https://doi.org/10.1016/j.smim.2014.01.008
Geissmann, F., Manz, M. G., Jung, S., Sieweke, M. H., Merad, M. and Ley, K. (2010) ‘Development of monocytes, macrophages, and dendritic cells’, Science, 327(5966), 656–661, available: https://doi.org/10.1126/science.1178331
Gordon, S. R., Maute, R. L., Dulken, B. W., Hutter, G., George, B. M., McCracken, M. N., Gupta, R., Tsai, J. M., Sinha, R., Corey, D., Ring, A. M., Connolly, A. J. and Weissman, I. L. (2017) ‘PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity’, Nature, 545, 495–499, available: https://doi.org/10.1038/nature22396
Gottfried, E., Kunz-Schughart, L. A., Ebner, S., Mueller-Klieser, W., Hoves, S., Andreesen, R., Mackensen, A. and Kreutz, M. (2006) ‘Tumor-derived lactic acid modulates dendritic cell activation and antigen expression’, Blood, 107(5), 2013–2021, available: https://doi.org/10.1182/blood-2005-05-1795
Gray, E. E. and Cyster, J. G. (2012) ‘Lymph node macrophages’, Journal of Innate Immunity, 4(5-6), 424–436, available: https://doi.org/10.1159/000337007
Guo, Z. S. (2018) ‘The 2018 Nobel Prize in medicine goes to cancer immunotherapy’, BMC Cancer, 18, 1086, available: https://doi.org/10.1186/s12885-018-5020-3
Gutiérrez-Martínez, E., Planès, R., Anselmi, G., Reynolds, M., Menezes, S., Adiko, A. C., Saveanu, L. and Guermonprez, P. (2015) ‘Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets’, Frontiers in Immunology, 6, 363, available: https://doi.org/10.3389/fimmu.2015.00363
Hernandez, C., Huebener, P. and Schwabe, R. F. (2016) ‘Damage-associated molecular patterns in cancer: a double-edged sword’, Oncogene, 35(46), 5931–5941, available: https://doi.org/10.1038/onc.2016.104
Hua, G. Y., Wang, P., Takagi, K., Shimozato, O., Yagita, H., Okigaki, T. and Matasumura, M. (1999) ‘Expression of a soluble form of CTLA4 on macrophage and its biological activity’, Cell Research, 9(3), 189–199, available: https://doi.org/10.1038/sj.cr.7290017
Hutten, T. J. A., Thordardottir, S., Fredrix, H., Janssen, L., Woestenenk, R., Tel, J., Joosten, B., Cambi, A., Heemskerk, M. H. M., Franssen, G. M., Boerman, O. C., Bakker, L. B. H., Jansen, J. H., Schaap, N., Dolstra, H. and Hobo, W. (2016) ‘CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses’, Journal of Immunology, 197(7), 2715–2725, available: https://doi.org/10.4049/jimmunol.1600011
Junt, T., Moseman, E. A., Iannacone, M., Massberg, S., Lang, P. A., Boes, M., Fink, K., Henrickson, S. E., Shayakhmetov, D. M., Di Paolo, N. C., van Rooijen, N., Mempel, T. R., Whelan, S. P. and von Andrian, U. H. (2007) ‘Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells’, Nature, 450, 110–114, available: https://doi.org/10.1038/nature06287
Junttila, I. S. (2018) ‘Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes’, Frontiers in Immunology, 9, 888, available:. https://doi.org/10.3389/fimmu.2018.00888
Kaiko, G. E., Horvat, J. C., Beagley, K. W. and Hansbro, P. M. (2008), ‘Immunological decision-making: how does the immune system decide to mount a helper T-cell response?’, Immunology, 123(3), 326–338, available: https://doi.org/10.1111/j.1365-2567.2007.02719.x
Kao, J. Y., Gong, Y., Chen, C.-M., Zheng, Q.-D. and Chen, J.-J. (2003) ‘Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine’, Journal of Immunology, 170(7), 3806–3811, available: https://doi.org/10.4049/jimmunol.170.7.3806
Kapellos, T. S.,Bonaguro, L., Gemünd, I., Reusch, N., Saglam, A., Hinkley, E. R. and Schultze, J. L. (2019) ‘Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases’, Frontiers in Immunology, 10, 1664-3224, available: https://doi.org/10.3389/fimmu.2019.02035
Ke, J., Yang, Y., Che, Q., Jiang, F., Wang, H., Chen, Z., Zhu, M., Tong, H., Zhang, H., Yan, X., Wang, X., Wang, F., Liu, Y., Dai, C. and Wan, X. (2016) ‘Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer’, Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine, 37(9), 12203–12211, available: https://doi.org/10.1007/s13277-016-5087-x
Khodarev, N. N., Roizman, B. and Weichselbaum, R. R. (2012) ‘Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth’, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 18(11), 3015–3021, available:https://doi.org/10.1158/1078-0432.CCR-11-3225
Kiertscher, S. M., Luo, J., Dubinett, S. M. and Roth, M. D. (2000) ‘Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells’, Journal of Immunology, 164(3), 1269–1276, available: https://doi.org/10.4049/jimmunol.164.3.1269
Kirby, A. C., Coles, M. C. and Kaye, P. M. (2009) ‘Alveolar macrophages transport pathogens to lung draining lymph nodes’, Journal of Immunology, 183(3), 1983–1989, available: https://doi.org/10.4049/jimmunol.0901089
Kobie, J. J., Wu, R. S., Kurt, R. A., Lou, S., Adelman, M. K., Whitesell, L. J., Ramanathapuram, L. V., Arteaga, C. L. and Akporiaye, E. T. (2003) ‘Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines’, Cancer Research, 63(8), 1860–1864, available: https://www.ncbi.nlm.nih.gov/pubmed/12702574
Kratofil, R. M., Kubes, P. and Deniset, J. F. (2017) ‘Monocyte Conversion During Inflammation and Injury’, Arteriosclerosis, Thrombosis, and Vascular Biology, 37(1), 35–42, available: https://doi.org/10.1161/ATVBAHA.116.308198
Lane, P., Gerhard, W., Hubele, S., Lanzavecchia, A. and McConnell, F. (1993) ‘Expression and functional properties of mouse B7/BB1 using a fusion protein between mouse CTLA4 and human gamma 1’, Immunology, 80(1), 56–61, available: https://www.ncbi.nlm.nih.gov/pubmed/8244464
Leirião, P., del Fresno, C. and Ardavín, C. (2012) ‘Monocytes as effector cells: activated Ly-6C(high) mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+ T cells’, European Journal of Immunology, 42(8), 2042–2051, available: https://doi.org/10.1002/eji.201142166
León, B., López-Bravo, M. and Ardavín, C. (2007) ‘Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania’, Immunity, 26(4), 519–531, available: https://doi.org/10.1016/j.immuni.2007.01.017
Leuschner, F., Dutta, P., Gorbatov, R., Novobrantseva, T. I., Donahoe, J. S., Courties, G., Lee, K. M., Kim, J. I., Markmann, J. F., Marinelli, B., Panizzi, P., Lee, W. W., Iwamoto, Y., Milstein, S., Epstein-Barash, H., Cantley, W., Wong, J., Cortez-Retamozo, V., Newton, A., Love, K., Libby, P., Pittet, M. J., Swirski, F. K., Koteliansky, V., Langer, R., Weissleder, R., Anderson, D. G. andNahrendorf, M. (2011) ‘Therapeutic siRNA silencing in inflammatory monocytes in mice’, Nature Biotechnology, 29(11), 1005–1010, available: https://doi.org/10.1038/nbt.1989
Lin, E. Y., Li, J.-F., Bricard, G., Wang, W., Deng, Y., Sellers, R., Porcelli, S. A. and Pollard, J. W. (2007) ‘Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages’, Molecular Oncology, 1(3), 288–302, available: https://doi.org/10.1016/j.molonc.2007.10.003
Louie, D. A. P. and Liao, S. (2019) ‘Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense’, Frontiers in Immunology, 10, 347, available: https://doi.org/10.3389/fimmu.2019.00347
Ma, J.F., Yang, F., Mahida, S.N., Zhao, L., Chen, X., Zhang, M.L., Sun, Z., Yao, Y., Zhang, Y.X., Zheng, G.Y., Dong, J., Feng, M.J., Zhang, R., Sun, J., Li, S., Wang, Q.S., Cao, H., Benjamin, E.J., Ellinor, P.T., Li, Y.G. and Tian, X.L. (2016) ‘TBX5 mutations contribute to early-onset atrial fibrillation in Chinese and Caucasians’, Cardiovascular research, 109(3), 442-450, available: https://doi.org/10.1093/cvr/cvw003
Mantovani, A., Allavena, P., Sica, A., and Balkwill, F. (2008) ‘Cancer-related inflammation’, Nature, 454, 436–444, available: https://doi.org/10.1038/nature07205
Marchetti, A., Di Lorito, A. and Buttitta, F. (2017) ‘Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle’, Journal of Thoracic Disease, 9(12), 4863–4866, available: https://doi.org/10.21037/jtd.2017.11.105
Martín-Fontecha, A., Lanzavecchia, A. and Sallusto, F. (2009) ‘Dendritic cell migration to peripheral lymph nodes’, Handbook of Experimental Pharmacology, 188, 31–49, available: https://doi.org/10.1007/978-3-540-71029-5_2
Menetrier-Caux, C., Montmain, G., Dieu, M. C., Bain, C., Favrot, M. C., Caux, C. and Blay, J. Y. (1998) ‘Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor’, Blood, 92(12), 4778–4791, available: https://doi.org/10.1182/blood.V92.12.4778
Mohamadzadeh, M., Berard, F., Essert, G., Chalouni, C., Pulendran, B., Davoust, J., Bridges, G., Palucka, A. K. and Banchereau, J. (2001) ‘Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells’, The Journal of experimental medicine, 194(7), 1013–1020, available: https://doi.org/10.1084/jem.194.7.1013
Moran, I., Grootveld, A. K., Nguyen, A. and Phan, T. G. (2019) ‘Subcapsular Sinus Macrophages: The Seat of Innate and Adaptive Memory in Murine Lymph Nodes’, Trends in Immunology, 40(1), 35–48, available: https://doi.org/10.1016/j.it.2018.11.004
Mosser, D. M. and Edwards, J. P. (2008) ‘Exploring the full spectrum of macrophage activation’, Nature Reviews Immunology, 8(12), 958–969, available: https://doi.org/10.1038/nri2448
Muntjewerff, E. M., Bianchi, F., Maassen, S. and van den Bogaart, G. (2019) ‘Measuring the molecular mechanisms of tumor antigen cross-presentation in dendritic cells and macrophages’, The Journal of Immunology, 202(1 Supplement), 177.7–177.7, available: https://www.jimmunol.org/content/202/1_Supplement/177.7.abstract
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., Gordon, S., Hamilton, J. A., Ivashkiv, L. B., Lawrence, T., Locati, M., Mantovani, A., Martinez, F. O., Mege, J.-L., Mosser, D. M., Natoli, G., Saeij, J. P., Schultze, J. L., Shirey, K. A., Sica, A., Suttles, J., Udalova, I., van Ginderachter, J. A., Vogel, S. N. and Wynn, T. A. (2014) ‘Macrophage activation and polarization: nomenclature and experimental guidelines’, Immunity, 41(1), 14–20, available: https://doi.org/10.1016/j.immuni.2014.06.008
Neumann, M., Murphy, N., and Seetharamu, N. (2022) ‘The Evolving Role of PD-L1 Inhibition in Non-Small Cell Lung Cancer: A Review of Durvalumab and Avelumab’, Cancer Medicine Journal, 5(1), 31-45. PMID: 35253011; PMCID: PMC8896901.
Noy, R. and Pollard, J. W. (2014) ‘Tumor-associated macrophages: from mechanisms to therapy’, Immunity, 41(1), 49–61, available: https://doi.org/10.1016/j.immuni.2014.06.010
Olingy, C. E., Dinh, H. Q. and Hedrick, C. C. (2019) ‘Monocyte heterogeneity and functions in cancer’, Journal of Leukocyte Biology, 106(2), 309–322, available: https://doi.org/10.1002/JLB.4RI0818-311R
Onishi, H., Morisaki, T., Baba, E., Kuga, H., Kuroki, H., Matsumoto, K., Tanaka, M. and Katano, M. (2002) ‘Dysfunctional and short-lived subsets in monocyte-derived dendritic cells from patients with advanced cancer’, Clinical Immunology, 105(3), 286–295, available: https://doi.org/10.1006/clim.2002.5293
Pardoll D. M. (2012) ‘The blockade of immune checkpoints in cancer immunotherapy’, Nature Reviews Cancer, 12(4), 252–264, available: https://doi.org/10.1038/nrc3239
Park, S. Y. and Kim, I. S. (2019) ‘Harnessing immune checkpoints in myeloid lineage cells for cancer immunotherapy’, Cancer letters, 452, 51–58, available: https://doi.org/10.1016/j.canlet.2019.03.018
Peranzoni, E., Lemoine, J., Vimeux, L., Feuillet, V., Barrin, S., Kantari-Mimoun, C., Bercovici, N., Guérin, M., Biton, J., Ouakrim, H., Régnier, F., Lupo, A., Alifano, M., Damotte, D. and Donnadieu, E. (2018) ‘Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment’, Proceedings of the National Academy of Sciences of the United States of America, 115(17), E4041–E4050, available: https://doi.org/10.1073/pnas.1720948115
Pico de Coaña, Y., Choudhury, A. and Kiessling, R. (2015) ‘Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system’, Trends in Molecular Medicine, 21(8), 482–491, available: https://doi.org/10.1016/j.molmed.2015.05.005
Santiago de Araújo Pio, C., Chaves, G., Davies, P., Taylor, R. and Grace, S. (2019) ‘Interventions to Promote Patient Utilization of Cardiac Rehabilitation: Cochrane Systematic Review and Meta-Analysis’, Journal of Clinical Medicine, 8(2), available: https://doi.org/10.3390/jcm8020189
Plosker, G. L. and Figgitt, D. P. (2003) ‘Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia’, Drugs, 63(8), 803–843, available: https://doi.org/10.2165/00003495-200363080-00005
Pozzi, L.-A. M., Maciaszek, J. W. and Rock, K. L. (2005) ‘Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells’, Journal of Immunology, 175(4), 2071–2081, available: https://doi.org/10.4049/jimmunol.175.4.2071
Pucci, F., Garris, C., Lai, C. P., Newton, A., Pfirschke, C., Engblom, C., Alvarez, D., Sprachman, M., Evavold, C., Magnuson, A., von Andrian, U. H., Glatz, K., Breakefield, X. O., Mempel, T. R., Weissleder, R., and Pittet, M. J. (2016) ‘SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions’, Science, 352(6282), 242–246, available: https://doi.org/10.1126/science.aaf1328
Qian, B.-Z., Li, J., Zhang, H., Kitamura, T., Zhang, J., Campion, L. R., Kaiser, E. A., Snyder, L. A. and Pollard, J. W. (2011) ‘CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis’, Nature, 475(7355), 222–225, available: https://doi.org/10.1038/nature10138
Richards, D. M., Hettinger, J. and Feuerer, M. (2013) ‘Monocytes and macrophages in cancer: development and functions’, Cancer Microenvironment: Official Journal of the International Cancer Microenvironment Society, 6(2), 179–191, available: https://doi.org/10.1007/s12307-012-0123-x
Rotte A. (2019) ‘Combination of CTLA-4 and PD-1 blockers for treatment of cancer’, Journal of experimental & clinical cancer research’, 38, 255, available: https://doi.org/10.1186/s13046-019-1259-z
Saikh, K. U., Khan, A. S., Kissner, T. and Ulrich, R. G. (2001) ‘IL-15-induced conversion of monocytes to mature dendritic cells’, Clinical and experimental immunology, 126(3), 447–455, available: https://doi.org/10.1046/j.1365-2249.2001.01672.x
Saio, M., Radoja, S., Marino, M. and Frey, A. B. (2001) ‘Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide’, Journal of Immunology, 167(10), 5583–5593, available: https://doi.org/10.4049/jimmunol.167.10.5583
Sambi, M., Bagheri, L. and Szewczuk, M. R. (2019) ‘Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates’, Journal of Oncology, 2019, available: https://doi.org/10.1155/2019/4508794
Savina, A., Jancic, C., Hugues, S., Guermonprez, P., Vargas, P., Moura, I. C., Lennon-Duménil, A.-M., Seabra, M. C., Raposo, G. and Amigorena, S. (2006) ‘NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells’, Cell, 126(1), 205–218, available: https://doi.org/10.1016/j.cell.2006.05.035
Seidel, J. A., Otsuka, A. and Kabashima, K. (2018) ‘Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations’, Frontiers in Oncology, 8, 86, available: https://doi.org/10.3389/fonc.2018.00086
Seoane, J. and Gomis, R. R. (2017) ‘TGF-β Family Signaling in Tumor Suppression and Cancer Progression’, Cold Spring Harbor Perspectives in Biology, 9(12), available: https://doi.org/10.1101/cshperspect.a022277
Shi, C. and Pamer, E. G. (2011) ‘Monocyte recruitment during infection and inflammation’, Nature Reviews Immunology, 11(11), 762–774, available: https://doi.org/10.1038/nri3070
Shi, Y., Fan, X., Deng, H., Brezski, R. J., Rycyzyn, M., Jordan, R. E., Strohl, W. R., Zou, Q., Zhang, N. and An, Z. (2015) ‘Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages’, Journal of immunology, 194(9), 4379–4386, available: https://doi.org/10.4049/jimmunol.1402891
Shiota, T., Miyasato, Y., Ohnishi, K., Yamamoto-Ibusuki, M., Yamamoto, Y., Iwase, H., Takeya, M. and Komohara, Y. (2016) ‘The Clinical Significance of CD169-Positive Lymph Node Macrophage in Patients with Breast Cancer’, PloS One, 11(11), available:. https://doi.org/10.1371/journal.pone.0166680
Shurin, M. R., Yurkovetsky, Z. R., Tourkova, I. L., Balkir, L. and Shurin, G. V. (2002) ‘Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10’, International Journal of Cancer, 101(1), 61–68, available: https://doi.org/10.1002/ijc.10576
Soudja, S. M., Ruiz, A. L., Marie, J. C. and Lauvau, G. (2012) ‘Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion’, Immunity, 37(3), 549–562, available: https://doi.org/10.1016/j.immuni.2012.05.029
Spary, L. K., Salimu, J., Webber, J. P., Clayton, A., Mason, M. D. and Tabi, Z. (2014) ‘Tumor stroma-derived factors skew monocyte to dendritic cell differentiation toward a suppressive CD14+ PD-L1+ phenotype in prostate cancer’ Oncoimmunology, 3(9), available: https://doi.org/10.4161/21624011.2014.955331
Tacken, P. J., de Vries, I. J. M., Gijzen, K., Joosten, B., Wu, D., Rother, R. P., Faas, S. J., Punt, C. J. A., Torensma, R., Adema, G. J. andFigdor, C. G. (2005) ‘Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody’, Blood, 106(4), 1278–1285, available: https://doi.org/10.1182/blood-2005-01-0318
Takeya, H., Shiota, T., Yagi, T., Ohnishi, K., Baba, Y., Miyasato, Y., Kiyozumi, Y., Yoshida, N., Takeya, M., Baba, H. and Komohara, Y. (2018) ‘High CD169 expression in lymph node macrophages predicts a favorable clinical course in patients with esophageal cancer’, Pathology International, 68(12), 685–693, available: https://doi.org/10.1111/pin.12736
Thepmalee, C., Panya, A., Junking, M., Chieochansin, T. and Yenchitsomanus, P.-T. (2018) ‘Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells’, Human Vaccines & Immunotherapeutics, 14(6), 1423–1431, available: https://doi.org/10.1080/21645515.2018.1431598
Toichi, E., Lu, K. Q., Swick, A. R., McCormick, T. S. and Cooper, K. D. (2008), ‘Skin-infiltrating monocytes/macrophages migrate to draining lymph nodes and produce IL-10 after contact sensitizer exposure to UV-irradiated skin’, The Journal of Investigative Dermatology, 128(11), 2705–2715, available: https://doi.org/10.1038/jid.2008.137
U. S. Food and Drug Administration (2022) Center for Drug Evaluation and Research
Vinogradov, S., Warren, G. and Wei, X. (2014) ‘Macrophages associated with tumors as potential targets and therapeutic intermediates’, Nanomedicine, 9(5), 695–707, available: https://doi.org/10.2217/nnm.14.13
Wculek, S. K., Cueto, F. J., Mujal, A. M., Melero, I., Krummel, M. F. and Sancho, D. (2020) ‘Dendritic cells in cancer immunology and immunotherapy’, Nature Reviews Immunology, 20(1), 7–24, available: https://doi.org/10.1038/s41577-019-0210-z
Wei, C., Yang, C., Wang, S., Shi, D., Zhang, C., Lin, X., Liu, Q., Dou, R. and Xiong, B. (2019) ‘Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis’, Molecular Cancer, 18(1), 64, available: https://doi.org/10.1186/s12943-019-0976-4
Wei, S. C., Duffy, C. R. and Allison, J. P. (2018) ‘Fundamental Mechanisms of Immune Checkpoint Blockade Therapy’, Cancer Discovery, 8(9), 1069–1086, available: https://doi.org/10.1158/2159-8290.CD-18-0367
Williams, L. M., Ricchetti, G., Sarma, U., Smallie, T. and Foxwell, B. M. J. (2004) ‘Interleukin-10 suppression of myeloid cell activation--a continuing puzzle’, Immunology, 113(3), 281–292, available: https://doi.org/10.1111/j.1365-2567.2004.01988.x
Yin, W., Gorvel, L., Zurawski, S., Li, D., Ni, L., Duluc, D., Upchurch, K., Kim, J., Gu, C., Ouedraogo, R., Wang, Z., Xue, Y., Joo, H., Gorvel, J.-P., Zurawski, G. and Oh, S. (2016) ‘Functional Specialty of CD40 and Dendritic Cell Surface Lectins for Exogenous Antigen Presentation to CD8(+) and CD4(+) T Cells’, EBioMedicine, 5, 46–58, available: https://doi.org/10.1016/j.ebiom.2016.01.029
Yokota, T., Otsuka, T., Mosmann, T., Banchereau, J., DeFrance, T., Blanchard, D., De Vries, J. E., Lee, F. and Arai, K. (1986) ‘Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities’, Proceedings of the National Academy of Sciences of the United States of America, 83(16), 5894–5898, available: https://doi.org/10.1073/pnas.83.16.5894
Yu, G. T., Bu, L. L., Zhao, Y. Y., Mao, L., Deng, W. W., Wu, T. F., Zhang, W. F. and Sun, Z. J. (2016) ‘CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma’, Oncoimmunology, 5(6), available: . https://doi.org/10.1080/2162402X.2016.1151594
Zappasodi, R., Merghoub, T. and Wolchok, J. D. (2018) ‘Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies’, Cancer Cell, 33(4), 581–598, available: https://doi.org/10.1016/j.ccell.2018.03.005
Zelenay, S. and Reis e Sousa, C. (2013) ‘Adaptive immunity after cell death’, Trends in Immunology, 34(7), 329–335, available: https://doi.org/10.1016/j.it.2013.03.005
Zhang, N. and Bevan, M. J. (2011) ‘CD8(+) T cells: foot soldiers of the immune system’ Immunity, 35(2), 161–168, available: https://doi.org/10.1016/j.immuni.2011.07.010
Zong, J., Keskinov, A. A., Shurin, G. V. and Shurin, M. R. (2016) ‘Tumor-derived factors modulating dendritic cell function’, Cancer Immunology, Immunotherapy: CII, 65(7), 821–833, available: https://doi.org/10.1007/s00262-016-1820-y