QIAOJING CHEN, KELLY TAN QIAN LIN, NICOLE SARAH YOW LI-WEN, SABRINA HENG CHER HUI, ALEXIS CHON MUN PING
ABSTRACT
Brain tumours are challenging to treat, partly because the blood-brain barrier (BBB) hinders targeted drug treatment. Patients diagnosed with aggressive brain tumours like glioblastoma multiforme (GBM) present low median survival of 15 months despite surgery with concurrent chemotherapy and radiotherapy. The poor prognosis and limited therapeutic effect have led to the exploration for an alternative intervention: nanotechnology. This paper focuses on nanotechnology-based diagnostic tools and drug delivery systems, such as multifunctioning nanoparticles that comprise an anti-cancer drug, an imaging agent and tumour specific ligands, which enhances diagnostic sensitivity and therapeutic efficacy against the malignant cells. Currently, the standard drug treatments have low specificity as they target both healthy and cancerous cells. Smaller particle size, manipulative surface properties and the capacity to conjugate with tissue recognition ligands or antibodies, nanoparticles have enhanced the transport of anti-cancer drugs across the BBB. However, certain limitations involving pharmacokinetics, toxic effects and uncertainty of chemical interactions between nanoparticles and the tumour microenvironment, drive the need for further research. Even though there are plenty of nanodrugs’ developments at present, not all obtained a preclinical proof as a result of failure in correlation between the in vitro and in vivo studies. The high complexity and arguable reproducibility are the main obstacles for clinical translation and regulatory approval. Yet, emerging evidence has shown that nanotechnology will potentially call forth a new era in diagnosis and treatment for brain tumours.
INTRODUCTION
According to the WHO classification of the central nervous system (CNS) tumours, glioblastoma multiforme (GBM) is a tumour of grade IV histological malignancy (Urbańska et al., 2014), and is the most aggressive and lethal form of brain tumour with a median survival of 15 months (Tzeng and Green, 2013). Approximately 24,000 new cases of primary malignant brain and CNS tumours are diagnosed annually and the number is estimated to grow (Becker and Baehring, 2017). There is currently no cure for GBM, and conventional treatments which include surgery, radiotherapy, chemotherapy and targeted drug therapy (Glaser et al., 2017), still result in an undesirable five-year survival rate of 4-5% (Carlsson et al., 2014). Thorough surgical resection of GBM is almost impossible as the tumour cells are highly infiltrative (Kim et al., 2014). The prognosis remains notably poor, mainly due to the tumour heterogeneity, uncontrolled proliferation, drug resistance and cancer recurrence (Carlsson et al., 2014).
The highly invasive nature of GBM with rapid proliferation, genetic heterogeneity and the unique microenvironment of the brain makes the treatment more challenging (Mendes et al., 2018). Current chemotherapeutics for GBM treatments involve the use of alkylating agents, mainly chloroethylating nitrosourea derivatives such as carmustine, nimustine and lomustine and the methylating agent temozolomide (TMZ) (Glaser et al., 2017; Iacob and Dinca, 2009). The gold standard of GBM treatment is concomitant chemotherapy using TMZ with radiotherapy (Stupp et al., 2005). While these alkylating agents have proven effectiveness in GBM treatments via cellular apoptosis, the limitations include hepatic and pulmonary toxicity (Fernandes et al., 2017) as well as non-targeted delivery of chloroethylating agents which may trigger apoptosis in healthy cells (Iacob and Dinca, 2009). Another major drawback of chemotherapy is the low bioavailability in the brain due to low permeability across the BBB (Nam et al., 2018). In addition, combination treatment of TMZ, re-irradiation and the addition of bevacizumab for patients with recurrent GBM, have also been found to cause neurologic toxicity (Fernandes et al., 2017).
In combination with carmustine, some clinical trials have also implemented the use of Gliadel wafers, an intracranially implanted biodegradable copolymer designed to achieve therapeutic efficiency with slow drug release (Iacob and Dinca, 2009; Silantyev et al., 2019). Despite increasing median survival by approximately 2 months in combination with radiation therapy (Ashby et al., 2016; Iacob and Dinca, 2009), Gliadel wafers may result in significant morbidities and fatalities by triggering seizures, intracranial infections, brain oedema, healing abnormalities, leaking of cerebrospinal fluid and formation of cysts (Iacob and Dinca, 2009; Kuramitsu et al., 2015). With the risk of nontargeted cytotoxicity and poor success rates due to inefficient drug delivery across the BBB, nanotechnology has been an area of interest in research studies to increase drug therapeutic windows and drug delivery efficacies for brain tumour treatments.
Gliomas are indistinguishable from other intracranial lesions like metastasis and abscesses (Pope and Brandal, 2018), making diagnosis and treatment a challenge. Medical imaging such as magnetic resonance imaging (MRI), computerized tomography (CT) and positron emission tomography (PET) are currently used to diagnose GBM. MRI is capable of achieving better contrast among soft tissues and is most commonly used in oncological imaging (Bhaskar et al., 2010). However, these imaging techniques are not sensitive enough to detect smaller GBM tumours (Alphandéry, 2018). Furthermore, MRI alone cannot differentiate between glioma grades, cell types and residual tumours from recurrent tumours (Pope and Brandal, 2018).
Nanotechnology and its associated possibilities were first framed by Nobel prize winner Richard P. Feynman in the 1950’s, to invite the scientists into the field of a tiny scale (Feynman, 1960). Nanoimaging and nanomedicine had advanced rapidly thereafter, especially in the application for cancer treatments. With the use of nanoparticles, medical imaging such as optical imaging and MRI are greatly enhanced. On the other hand, the versatility of nanoparticles drives the establishment of research not only on cancer treatment, but also on other diseases such as neurodegenerative diseases, HIV/AIDS, ocular diseases and respiratory diseases (Murthy, 2007). Nanoparticles can be administered via oral, inhaled, intravenous or transdermal routes. Among the various routes of administration, intravenous delivery is the most common drug delivery method for GBM, which adopts two targeting mechanisms: passive and active targeting (Chenthamara et al., 2019). Passive targeting refers to the ability of nanoparticles to diffuse across the tumour blood vessels that are highly permeable, and accumulate within neoplastic tissues; whereas active targeting allows selective binding of drug molecules to specific receptors on tumour cells by utilising ligand-conjugated nanoparticles.
Nanoparticles constructed for brain tumour therapy are mainly made of gold-based, silver-based, magnetic, lipid-based, dendrimeric and polymeric nanomaterial. Multi-functional nanoparticles, commonly defined as having three external dimensions at a nanoscale of no more than 100 nm in diameter (Jeevanandam et al., 2018), have potentially initiated a new era for brain imaging. Owing to these dynamic nanoparticles, tumour development can be monitored at a molecular and cellular level. Nanodiagnostics such as MRI involving dendrimers and metallic nanoparticles have resulted in increased sensitivity and accuracy in the detection of tumours (Mendes et al., 2018). Early diagnosis is critical in improving survival rates since better treatment efficacies might be achieved for smaller tumours, which is now achievable using nanoparticles.
Nanotheranostics, the integration of diagnosis with targeted treatment, has shown promising results as a great medical enhancement in oncology (Zottel et al., 2019). Nanoparticles exhibit many enhanced properties like smaller size, higher surface area to volume ratio, reactive surfaces, and improved bioavailability (Zottel et al., 2019), which help to overcome BBB restrictions, delay drug metabolism and thus enabling controlled and specific release of the drug (Mendes et al., 2018). They can be manipulated using established nanotechnology techniques to conjugate with tissue recognition ligands or antibodies to increase tumour specificity and improve pharmacokinetics for overall treatment efficacy. The encapsulated chemotherapeutic agent can also be made safer for the patient by modifying the nanoparticle with biocompatible polymers. Along with attachment of diagnostic molecules such as a contrast agent or a fluorescent macromolecule, precise delineation of brain tumours and possible drug therapeutic effects on tumour cells can be determined (Sonali et al., 2018). Nanotechnology has thus stimulated interests for detailed investigations to provide more selective therapeutic treatments as well as enhancements in diagnostic imaging for brain tumours (Becker and Baehring, 2017; Mangraviti et al., 2016). In this paper, we review the recent developments of nanoparticle-based diagnostic tools and drug delivery systems. We highlight the major obstacles in the past, unique properties of each nanoparticle, animal models, clinical trials, limitations in application and prominent prospects of nanotechnology.
METHODS
In this report, a comprehensive research of peer-reviewed journal articles and literature reviews between 2002-2021 from data sources: The National Centre for Biotechnology Information (NCBI), ScienceDirect, Scopus and the LTU library was performed. Journals from Asia, America and Europe were used.
DRUG TRANSPORT ACROSS BRAIN BARRIERS
Blood-Brain Barrier (BBB)
The BBB is a physical, metabolic and immunological barrier preventing drugs and neurotoxic substances from being transported into the brain. There are several transport pathways depending on physicochemical properties that can be exploited to transport drugs across the BBB.
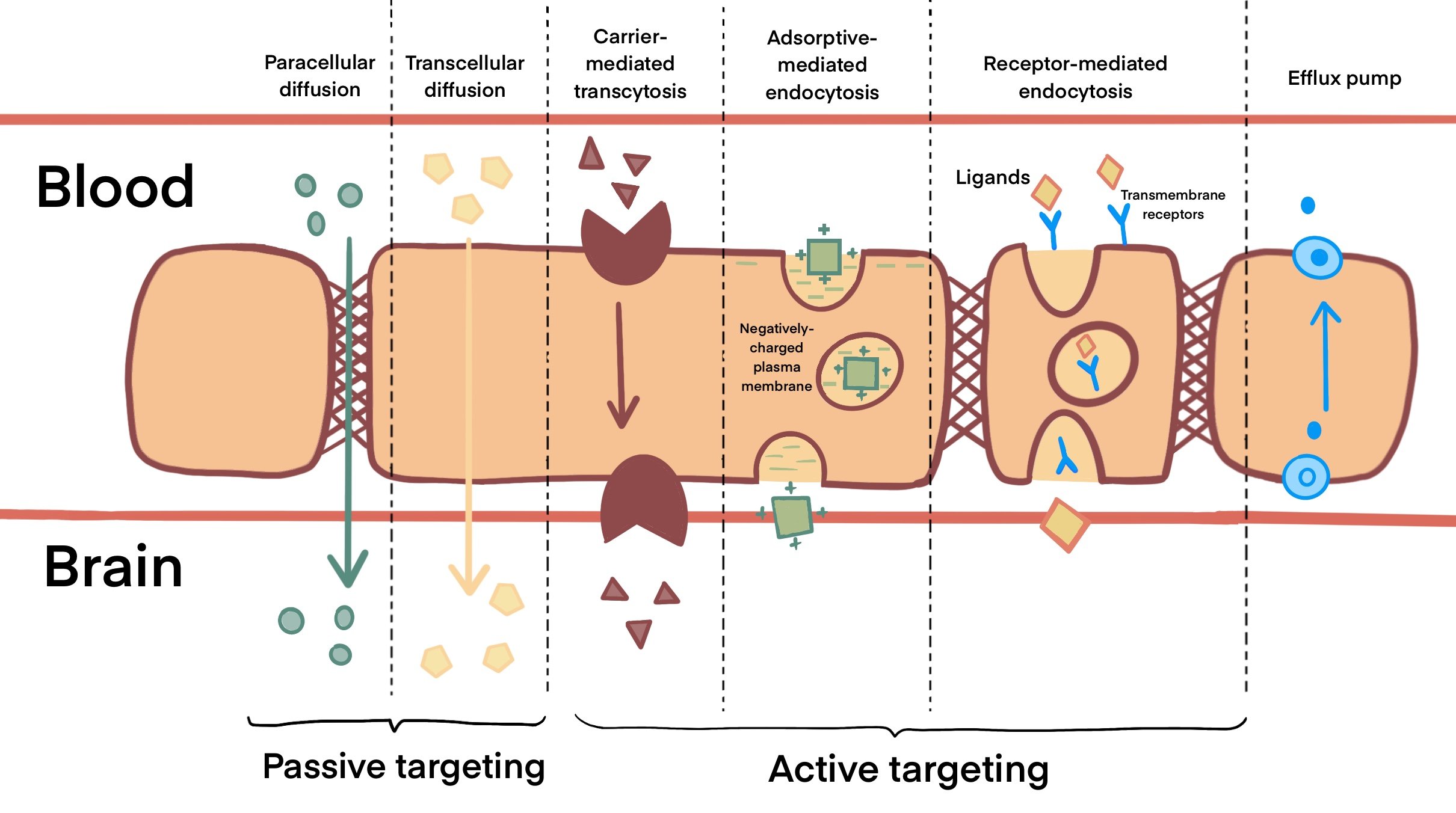
As shown in Figure 1, carrier-mediated transcytosis (CMT) internalizes small molecules like glucose, hormones and amino acids by passive diffusion across the BBB (Wei et al., 2014). This can be investigated for improved BBB transport using drugs that mimic these carrier substrates. In contrast, adsorptive-mediated transport (AMT) carries macromolecules in a membrane-bound vesicle between polarized cells (Hervé et al., 2008). These cationic molecules are able to interact with the anions surrounding BBB endothelial cells. However, surface charge can alter the interaction between nanoparticles and cells by affecting the cellular uptake, biodistribution, metabolism and excretion. Unlike CMT and AMT, receptor-mediated transcytosis (RMT) enables cells to endocytose a molecule when a ligand binds to a receptor on the membrane of an endothelial cell (Thuenauer et al., 2017). However, this method of transport is also present in other parts of the body such as the intestines and liver, hence it can be less selective in targeting the brain tumour (Mendes et al., 2018).
Figure 1. Transport mechanisms across the BBB. Passive targeting across the BBB involves paracellular and transcellular diffusion of small, highly lipophilic molecules and nanoparticles that are smaller than 10nm in size. Molecules that are unable to diffuse through the BBB, such as drugs used for active targeting, are facilitated across the BBB by carrier-mediated transcytosis, adsorptive-mediated endocytosis or receptor-mediated endocytosis to reach the tumour site. In addition, active efflux pumps expressed at the BBB hinder the drug accumulation and reduce the effect of chemotherapeutics as they remove the drug from the CNS.
Blood-Brain-Tumour Barrier (BBTB)
The BBTB is a network of tumour vessels that delivers nutrients to tumours and aids in tumour migration. In the initial phase of GBM, the BBTB usually remains intact. However, as the tumour progresses to the second and third phase, the BBTB will be compromised with increasing permeability. By the third phase, gaps are formed between cerebral endothelial cells and it allows molecules up to one micron to enter the brain (Mendes et al., 2018; van Tellingen et al., 2015). There are two important approaches that could be exploited to target and accumulate nanoparticles in tumour tissues: passive targeting by enhanced permeability and retention (EPR) effect and active targeting by binding conjugated ligands to the receptors of tumour cells (Nam et al., 2018) (Teleanu et al., 2018a).
Passive Targeting for GBM
Passive targeting, which was first described by Maeda and Matsumura (Maeda and Matsumura, 1989; Matsumura and Maeda, 1986), refers to the ability of nanoparticles to diffuse across vulnerable tumour blood vessels toward neoplastic tissues and preferentially accumulate at the area as a result of the EPR effect. The permeability of tumour blood vessels is higher with pore sizes ranging from 380-780 nm, in contrast to healthy blood vessels that are 4-25 nm (Nam et al., 2018). However, nanoparticles smaller than 10 nm have exhibited shorter circulation time compared to nanoparticles that are 10-100 nm in size. Meanwhile, lymphatic drainage is usually impaired in the case of brain tumour, which increases the retention time for accumulated nanoparticles at the tumour site. Studies have shown that nanoparticles ranging from 100-400 nm in size had no significant differences in crossing the BBB as compared to smaller nanoparticles. The ideal nanoparticle size is suggested to be between 10-100 nm (Danhier, 2016; Nam et al., 2018).
Active Targeting for GBM
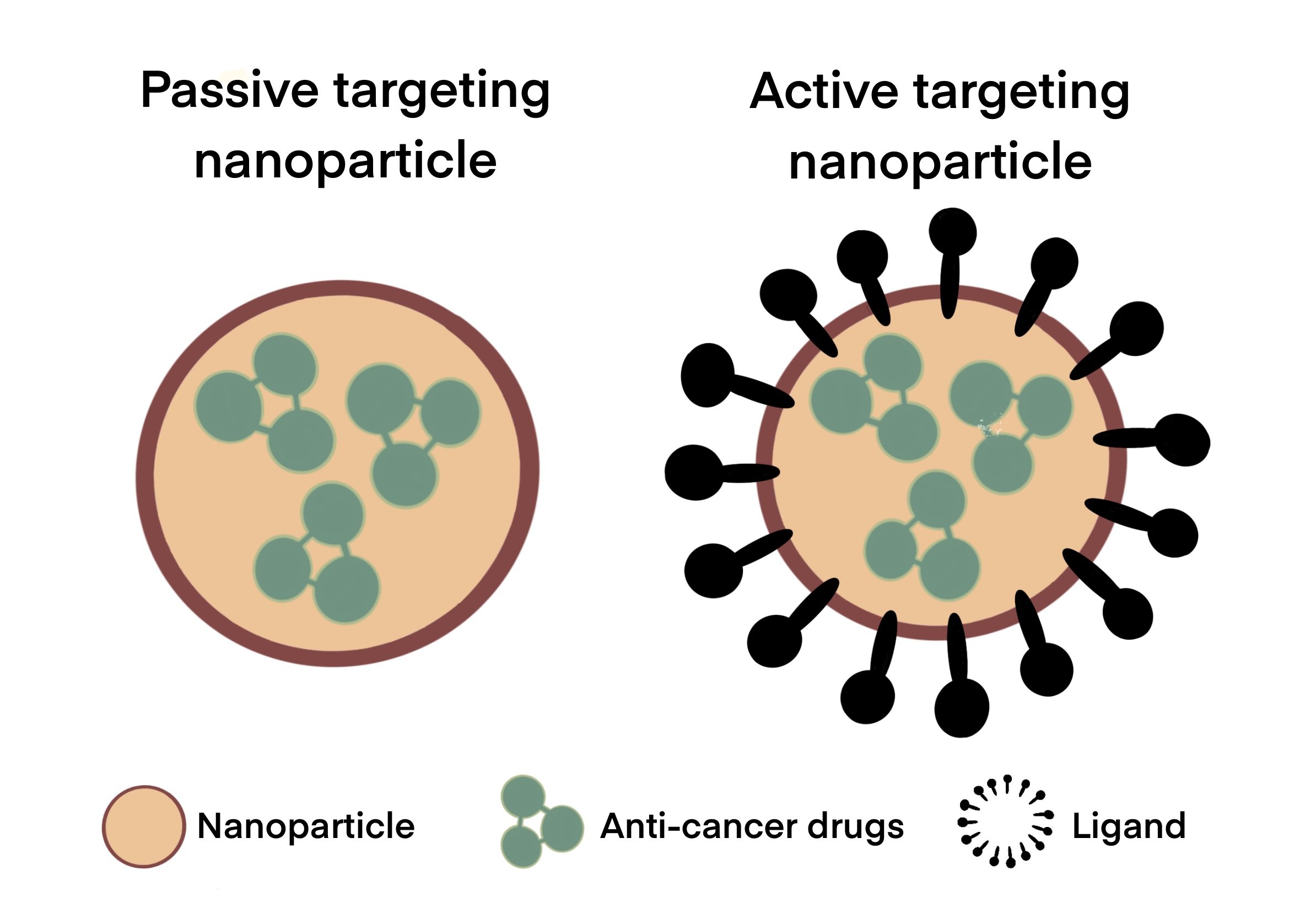
As shown in Figure 1, active targeting improves uptake selectivity of anti-cancer drugs. The difference between the nanoparticles involved in passive and active targeting is illustrated in Figure 2. By binding to the specific receptors that are overexpressed in tumour cells, ligand-conjugated nanoparticles can selectively target cancer cells without damaging healthy tissue (Mendes et al., 2018). This improves the therapeutic efficacy of anti-cancer drugs, thereby decreasing potential negative side effects. In GBM, this also includes targeting specific proteins on the BBB capillaries that can transfer nanoparticles from the blood to the brain (Gao, 2017). A study which had compared active and passive targeting by adding an epidermal growth factor receptor (EGFR)-targeting peptide to one nanoparticle for active targeting, and another without EGFR for passive targeting, showed that active targeting had boosted the efficacy of nanoparticles in cancer treatment (Clemons et al., 2018). Active targeting nanomaterials also include stimuli-responsive carriers that can release therapeutic agents in response to internal or external stimuli such as pH and temperature (Shim and Kwon, 2012). Many studies that had adopted an active targeting approach have designed nanoparticles with dual targeting properties to improve their ability in penetrating the BBB and enhancing uptake by tumour cells (Nam et al., 2018).
Figure 2. Comparison between nanoparticles involved in passive and active targeting. Nanoparticles (e.g. liposomes, dendrimers, polymeric nanocarriers, etc.) carrying various anti-cancer drugs can cross the BBB either by passive or active targeting. Passive targeting involves diffusion across the BBB while active targeting involves the conjugation of ligands (eg. proteins, carbohydrates, oligonucleotides, etc.) to nanoparticles for higher specificity and uptake of drug at the tumour site.
Despite multiple transport pathways across the BBB, it still presents a major obstacle in drug delivery due to poor permeability of anti-cancer drugs. Drugs that are able to diffuse across the BBB via passive transport may also be actively transported back into circulation by efflux pumps (e.g. P-glycoprotein) that are expressed on the endothelial surface of the BBB (Harder et al., 2018). Hence, systemically administered drugs are often hindered from reaching its desired target location, resulting in low, ineffective drug concentration and continuous poor prognosis for most glioblastoma cases (Zottel et al., 2019). Among other methods to enhance drug delivery, direct injection and arterial injection of osmotic solutions are often invasive, hard to repeat and may lead to undesirable consequences such as risk of infections or brain trauma. Paired with a circulating microbubble agent, focused ultrasound (FUS) is one of the repeatable physical methods used in contrast-enhanced MRI that has shown a positive outcome with good selectivity for the opening position and subsequent increase in BBB permeability. However, the increase in BBB permeability had resulted in bleeding and heat coagulation injury at the opening sites (He et al., 2018). Hence, the limitations presented by current available methods propel the need for further research on alternative strategies to mediate drug delivery through the BBB.
NANOPARTICLES FOR BRAIN TUMOUR THERAPY
With a variety of nanocarrier compositions, conjugated ligand designs, surface characteristics and particularly small size, both inorganic and organic nanoparticles have been the focus of drug delivery research in brain tumour treatments as they oppose the challenges presented by the BBB and are less invasive (Nam et al., 2018).
Inorganic nanoparticles are able to absorb large amounts of X-ray due to their high atomic number. Combining targeted therapy based on specific targeted diagnostic tests, the increase in radio enhancement effects and physical processes improve the capacity of metallic nanoparticles as theranostic agents. This makes them a preferred choice for radio-sensitization, radiation therapy and diagnostic imaging (Pinel et al., 2019). In contrast, organic nanoparticles are able to incorporate biocompatible materials like hydrophilic polymers, hydrogels and dendrimers that add flexibility to the overall structure, allowing more modifications for drug-loading and release (Meyers et al., 2013).
Gold-Based Nanoparticles
With core sizes ranging from 1-150 nm, gold nanoparticles have shown promising results in drug delivery systems and as theranostic agents due to their capacity for surface plasmon resonance, ease of antibody conjugation (Singh et al., 2018), biocompatibility and quenching efficiencies (Farooq et al., 2018). Upon accumulation at tumour sites and radiated with wavelengths between 800-1200 nm, gold nanoparticles promote cancer cell apoptosis by releasing heat that denatures proteins, nucleic acids and cellular membranes. Gold nanoparticles also display anti-angiogenic properties as they are capable of blocking vascular endothelial growth factor (VEGF). The VEGF receptor binding hinders downstream phosphorylation and activation of the PI3K/Akt signalling pathway, which is required for cellular metabolism, proliferation, growth, survival and angiogenesis (Hemmings and Restuccia, 2012). In addition, cellular internalization of gold nanoparticles induce activation of caspase-9 which promotes apoptosis (Chugh et al., 2018). They can also be engineered to carry anti-cancer drugs across the BBB that are released upon irradiation.
Application of Gold-Based Nanoparticles
Methotrexate, a chemotherapy drug which does not readily penetrate the BBB, had shown an increase in cytotoxicity and tumour uptake when conjugated with gold nanoparticles, as compared to free methotrexate (Singh et al., 2018). The accumulation of gold nanoparticles at tumour sites was supported by Hainfeld et al., who had produced a 19:1 ratio of mouse glioma-to-healthy tissue localization of gold nanoparticles. Subsequently, CT scans had obtained a higher resolution of the tumour image as compared to conventional imaging without nanoparticle radio-enhancers (Pinel et al., 2019).
Specificity for tumour cells can be enhanced by conjugating the nanoparticle with an antibody or ligand (Zottel et al., 2019). In another study, gold nanoparticles conjugated with transferrin peptide (Tfpep or Tf) were designed to load the photodynamic pro-drug, phthalocyanine 4 (Pc 4), to better target brain glioma cells (Dixit et al., 2015). Transferrin, one of the key elements in ensuring normal neurological activities of the brain, is a transport protein that imports iron across the BBB via transferrin receptors (TfR). In GBM, tumour progression is facilitated by iron accumulation as a result of overexpression of TfR, which is the most well-known endothelial receptor for brain drug delivery via RMT (Qian et al., 2002). The efficiency and specificity to deliver Pc 4 drug has been improved by adopting the Tfpep conjugated gold nanoparticles, as compared to the unconjugated gold nanoparticles or the free drugs. As observed with fluorescence imaging, in vivo mice studies have exhibited a greater drug uptake, achieving 1/10th of the standard of care (SOC) therapeutic dose of free Pc 4 (Dixit et al., 2015). Conjugation of Tfpep also minimizes both the exposure of healthy tissues to the toxic metal ions and off-site accumulation of Pc 4. In addition, surface modification of gold nanoparticles by attachment of ligands such as antibodies, Tf, fibroblast growth factors and low-density lipoproteins, not only enhances penetration across the BBB, but have also been found to increase the subcellular imaging of tumours using multiphoton and scanning electron microscopy (Mukhtar et al., 2020). Thus, gold nanoparticles are multifunctional agents perfect for both combined therapy and diagnostic applications, possessing enhanced radio-sensitizing effects for both in vivo and in vitro imaging as well as thermal ablation of tumours (Farooq et al., 2018).
Although gold nanoparticles are known for their inert nature and lack of toxicity (Kaul et al., 2018; Singh et al., 2018), there have been few reports on the toxicity of this nanomaterial mostly due to physical dimensions (Farooq et al., 2018; You et al., 2014) like surface-to-volume ratio which may promote catalytic properties, increasing its reactivity with the environment (Gerber et al., 2013).
Silver-Based Nanoparticles
Synthesis of silver nanoparticles can be done via physical, chemical and biological methods such as laser ablation, thermal decomposition, chemical reduction and/or microorganism synthesis (De Matteis et al., 2018). First applied for their antibacterial properties, silver nanoparticles are now frequently investigated for their applications in cancer diagnosis and drug delivery systems due to their excellent thermal conductivity, plasmon resonance effects and chemical stability (Teleanu et al., 2018b) (Pinel et al., 2019). The stability of the silver core can be further improved by applying a coat of citrate or layer of polyvinylpyrrolidone (PVP) , increasing drug bioavailability in circulation (Zottel et al., 2019). In addition, silver nanoparticles also exhibit radiosensitizing effects which hinders DNA duplication and repair mechanisms of cancer cells, resulting in apoptosis (Glaser et al., 2017). Similar to gold-based nanoparticles, silver nanoparticles also display anti-angiogenic effects as they inhibit phosphorylation of Akt in the PI3K/Akt signalling pathway (Chugh et al., 2018).
Application of Silver-Based Nanoparticles
Hsin and his colleagues have reported that the uptake of silver nanoparticles had disrupted mitochondrial cellular respiration and produced reactive oxygen species which resulted in cancer cell apoptosis (Hsin et al., 2008). Furthermore, a 5-fold survival rate was achieved for the C6 glioma-bearing mice, after administering 10 or 20 mg of citrate-capped silver nanoparticles with concurrent 10 Gy-radiotherapy, and no significant systemic toxicity was reported (P. Liu et al., 2013). Silver nanoparticles possess intrinsic properties which allow easy penetration and uptake by mammalian cells via energy-driven internalization pathways. Upon selective uptake into tumour cells, these silver nanoparticles can be detected by their specific fluorescence in x-ray irradiation applications. Moreover, silver nanoparticles possess plasmonic features involving the scattering and absorbance of light which are useful for both diagnostic imaging purposes as well as tumour selective hyperthermia, making them a winning option as theranostic agents (Burdus, el et al., 2018).
However, various toxicology responses have been reported in other studies with in vitro use of silver nanoparticles, largely due to the size, coating, amount of exposure, interaction with the microenvironment and its administered concentration (De Matteis et al., 2018). The toxicology profiles involving the use of silver nanoparticles as drug-delivery systems and for diagnostic applications would need to be further investigated.
Magnetic Nanoparticles
Magnetite (Fe3O4), hematite (α-Fe2O3) and maghemite (Fe2O3) are iron oxides that usually make up the core of magnetic nanoparticles (Pinel et al., 2019). As magnetic nanoparticles are targeted by the reticuloendothelial system, coating with materials such as polysaccharide, polymers, lipid and protein can prevent rapid degradation and increase its stability in vivo. Magnetic nanoparticles can be categorized as paramagnetic nanoparticles (PMNPs) or superparamagnetic iron oxide nanoparticles (SPIONs). SPIONS are most commonly studied due to their optical and magnetic properties which are more enhanced than PMNPs. In addition, they have longer tumour retention periods with adequate degradation for effective treatment (Thomsen et al., 2015). SPIONS have zero magnetization without the presence of magnetic fields but can be delivered to the tumour site by applying an external magnetic field. However, this depends on particle size and usually only applies to SPIONs that are 10-20 nm as they exist as a single magnetic domain with higher magnetic properties as compared to larger magnets with multiple domains (Wahajuddin and S, 2012). In magnetic hyperthermia, cancer cells are destroyed with alternating magnetic fields at approximately 41-50 ◦C (Pinel et al., 2019; Zottel et al., 2019). Particles that are less than 100 nm with neutral or negatively charged surfaces are the most ideal in targeting brain tumour across the BBB (Wankhede et al., 2012).
Application of Magnetic Nanoparticles in Drug Delivery Systems
To increase binding specificity and internalization, the particles can be conjugated with EGFR which are overexpressed in about 50% of GBM cases (Hatanpaa et al., 2010). Drug delivery can be enhanced by encapsulating the drug-loaded SPION within a liposome to facilitate its transport across the BBB. In another study, magnetic silica PLGA nanoparticles were conjugated with Tf to mediate the transport of nanoparticles across BBB and enhance drug accumulation in the brain tumour. Anti-cancer drugs doxorubicin (DOX) and paclitaxel (PTX) were loaded in these nanoparticles. With a smaller tumour size detected in the experimental mice, these dual drug nanoparticles displayed a more competent anti-glioma activity compared to the free DOX and/or PTX (Cui et al., 2013).
Application of Magnetic Nanoparticles in Diagnostic Imaging
Silica-coated magnetic iron oxide nanoparticles have been shown to generate high accuracy in vivo fluorescence visualization of GBM tumours under near-infrared fluorescence, as well as in vitro and in vivo fluorescence imaging of tumour-associated macrophages. These water-soluble silica-coated nanoparticles are also favourable as multimodal contrast agents for imaging. Thus, silica-based iron oxide nanoparticles make great candidates for diagnostic imaging due to their capability to provide sensitive and precise delineation of GBM tumours (Mukhtar et al., 2020).
However, some obstacles faced by magnetic nanoparticles include obtaining optimal temperature heating within the tumour volume for effective and safe treatment as well as the risk of generating reactive oxygen species (ROS) which may cause apoptosis in normal cells (Thomsen et al., 2015; Zottel et al., 2019). Moreover, iron overload resulting in high levels of bare Fe2O3 nanoparticles may cause DNA damage/mutations, unwanted inflammatory responses and disruption to cellular cytoskeletal arrangements. A detailed investigation of its pharmacokinetics would be beneficial as the shape, size and surface properties of SPIONs involved in drug delivery systems may affect its biodistribution as well as development of SPION-associated toxicities (Wahajuddin and S, 2012).
Lipid-Based Nanoparticles
Lipid-based nanocarriers consist of liposomes, solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC).
Liposomes
Made up of amphipathic phospholipid bilayers, liposomes form vesicles in water and are capable of encapsulating both hydrophobic and hydrophilic drugs. While the stability of the liposomes can be improved with cholesterol or phosphatidylcholine coating, the biocompatibility, half-life and water solubility of the vesicles can be enhanced with polyethylene glycol (PEG) coating (García-Pinel et al., 2019). Exploiting the EPR effect, liposomes can be endocytosed and engineered to release the drug into the tumour space upon favourable conditions depending on pH, redox or the presence of an electromagnetic field. They can also be conjugated with antibodies or specific ligands to enhance active targeting of tumour cells (Ying et al., 2010). In one of the studies, peptide RDP-modified nanoliposomes (RCL), which could be endocytosed by glioma cells via acetylcholine receptors, were developed to deliver anti-cancer drug curcumin to the brain. The experimental results indicated that the RCL allowed stable release of curcumin and prolonged the life span of the glioma-bearing mice from 23 to 33 days (Zhao et al., 2018)
Solid Lipid Nanoparticles (SLN)
Unlike liposomes, a composition of glycerides, fatty acids and mono-, di- or triglycerides allow SLNs to remain at a solid state at both body and room temperatures. SLNs are mostly known for the controlled release of drugs, lower toxicity, modifications for specific targeting as well as further stabilization with a surfactant or polymer coating (García-Pinel et al., 2019). However, the inevitable development and unfavourable enhancement of the SLN crystal structure during the production and storage process may risk expulsion of the encapsulated drug and thus result in inefficient drug loading (Duan et al., 2020; Ghasemiyeh and Mohammadi-Samani, 2018).
Nanostructured Lipid Carriers (NLC)
NLCs are a second generation of lipid nanocarriers designed to overcome this early release of the loaded drug, which is a huge limitation of SLNs (Duan et al., 2020). In contrast to SLNs, NLCs comprise both solid and liquid lipids and a less than perfect crystal structure as compared to SLNs. This reduces drug expulsion and enhances its drug loading capacity. The presence of liquid lipids enhances solubility and controlled drug release into the lipid matrix (Ghasemiyeh and Mohammadi-Samani, 2018).
Application of Lipid-Based Nanoparticles
While the ease of passage across the BBB is provided by its lipidic characteristic, lipid nanocarriers are also suitable candidates as theranostic agents as they can be conjugated with both chemotherapeutic and imaging agents. One such example would be docetaxel-coated D-alpha-tocopheryl PEG-1000 succinate mono-ester (TPEGS) coated liposomes, conjugated with Tf for active targeting and co-loaded with quantum dots (QD) for tumour imaging. The results have shown an increase in uptake and greater fluorescence intensity from the modified liposomes as compared to unconjugated liposomes. In addition, QD-loaded liposomes present higher permeability and retention rate in the brain as compared to free QD, aiding in tumour imaging applications (Bibhash et al., 2019).
Overall, lipid nanocarriers possess many features such as being able to incorporate both hydrophilic and lipophilic drugs to enhance drug uptake and the surface modifications allow loading of imaging agents for simultaneous diagnosis and treatment of GBM (Bibhash et al., 2019).
Dendrimers
Polyamidoamine (PAMAM) dendrimers have been frequently investigated in treatment strategies for brain diseases due to its capacity for slow drug release, low toxicity and increased tumour uptake (Zottel et al., 2019). Made up of repetitive layers of synthetic macromolecules, dendrimers comprise a core where drugs can be encapsulated. Surface modification and attachment of contrast agents, functional groups, antibodies or homing peptides enable diverse functionalization (Teleanu et al., 2018a). Tumour internalization of PAMAM dendrimers can be enhanced by conjugation with PEG and glioma-specific ligands which increases transfection efficiency and drug delivery (Jiang et al., 2016). In addition, tumour distribution of nanodrugs can be increased with dual targeting using a BBB-targeting peptide such as Angiopep, along with a specific aptamer for active tumour targeting (Meyers et al., 2013). However, generation 2-4 dendrimers are unable to exploit the EPR effect due to its minute size. The surface of dendrimers are also toxic, but can be coated with PEG to reduce toxicity and increase its biocompatibility (Zottel et al., 2019).
Application of Dendrimers
Dual targeting PEGylated PAMAM dendrimers conjugated with tumour targeting folic acid (FA) and borneol (BO) have been synthesized to improve the permeability of DOX across the BBB. FA conjugation led to 3-fold lower IC50 (i.e. half maximal inhibitory concentration) value and BO modification boosted BBB permeability two fold. These dendrimers had significantly extended the half-life and boosted DOX accumulation in tumour tissues, as compared to free DOX. The in vivo tumour growth was inhibited by up to an additional 57.4%. The median survival time of the xenograft mouse increased from 18 to 28 days (Xu et al., 2016). Dendrimers can also be used in diagnosis of GBM as an MRI contrast agent. Gd(III)-DTPA is a commonly used MRI contrast agent that has great contrast enhancement, however, it accumulates in the liver due to slow excretion rate (Noriega-Luna et al., 2014). By linking Gd(III)-DTPA to PAMAM dendrimers, the relaxation rate of in vivo water protons can be enhanced, resulting in minimized doses of Gd ions that cause metal ion toxicity, as well as an improvement in image contrast enhancement (Longmire et al., 2008).
Polymeric Nanocarriers
Polymers exhibit pharmacokinetic and pharmacodynamic characteristics that make them ideal as drug delivery systems. While polylactic-co-glycolic acid (PLGA) has already been approved for use by the Food and Drug Administration (FDA), polybutylcyanoacrylate (PBCA) has also been frequently studied in drug delivery applications due to their biodegradability, biocompatibility and lack of toxicity (Saucier-Sawyer et al., 2015; Zottel et al., 2019). PBCA can be engineered to release the drug via surface desorption, polymer matrix degradation, diffusion from the matrix or wall, or a combination of both. In order for excretion of non-biodegradable polymer nanocarriers, an estimated diameter of 5-6 nm would be most ideal for renal filtration. In contrast, most biodegradable polymeric nanocarriers have been well-tolerated in clinical trials and a recent study has seen improvements for brain tumours following gene therapy using polymeric nanocarriers. Moreover, DOX, camptothecin and PTX have been able to cross the BBB to reach the tumour site using polymeric nanodrugs (Meyers et al., 2013).
Application of Polymeric Nanocarriers
In one study, epirubicin (Epi), a potent anti-glioblastoma drug, was loaded in cylic-Arg-Gly-Asp (cRGD) -conjugated polymeric micelles to investigate the treatment of GBM. Both free Epi and these nanodrugs (cRGD-Epi/m) were injected intravenously to mice. Free Epi has a very minor effect on the suppression of GMB on orthotopic xenografts. cRGD-Epi/m showed a 12-fold lower photo response caused by the tumour cells after 20 days. cRGD-Epi/m effectively suppressed the growth of orthotopic GBM model by delivering high levels of epirubicin throughout the tumour tissue (Quader et al., 2017).
Furthermore, a recent study on tumour uptake of red fluorescent carbonized polymer dots have reported substantial internalization in glioma cells with low toxicity and photochemical degradation as well as long emission and excitation wavelengths. Polymeric nanocarriers are thus able to enhance the MRI quality of gliomas and aid in visualization of real-time surgery as nanoprobes. Photostable polymeric nanocarriers that have the ability to absorb near-infrared beams can also offer accurate photoacoustic imaging when modified with ligands for active targeting of tumour cells (Mukhtar et al., 2020).
However, polymers are often targeted by opsonization in the blood and would be rapidly cleared from the body unless a protective coating such as hyperbranched polyglycerol is applied (Saucier-Sawyer et al., 2015). Although promising in brain tumour targeting and precise intraoperative imaging, long-term effects of polymers and possible neurotoxicity are yet to be confirmed due to insufficient information available (Meyers et al., 2013).
Comparison Between the Nanoparticles
Different types of nanoparticles exhibit unique features that can be utilised to improve the in vivo performance of drugs or imaging. While each type of nanoparticles can be functionalized to improve diagnostic or therapeutic efficacies, multifunctional nanoparticle complexes are also designed to integrate various features of materials to achieve an enhanced effect and higher tunability. A multifunctional nanoparticle complex normally has a core that targets or aids in diagnostic imaging (with materials such as gold, magnetic or SPIO) at the tumour site. The therapeutic or diagnostic cargo can also be attached to the nanoparticle’s surface (Ventola, 2012). Both gold and silver nanoparticles have exhibited promising therapeutic efficacies. However, they are not biodegradable and might exhibit a range of toxicities against healthy cells depending on the shape, surface-to-volume ratio and the reactivity of the nanoparticle to the microenvironment. With excellent thermal conductivity and surface plasmon resonance, both metallic nanoparticles are effective in photothermal and photodynamic therapies against cancer cells. In addition, both gold and silver nanoparticles possess anti-angiogenic and anti-proliferative properties as they can block, disrupt, and hinder the PI3K/Akt signalling pathway at different checkpoints. However, unlike gold nanoparticles which have been found to be less toxic and more biocompatible, silver nanoparticles pose greater risks of toxicity against normal cells due to its cellular uptake via endocytosis and intracellular trafficking. Upon internalization and translocation to the mitochondria and nucleus, silver nanoparticles stimulate a series of downstream responses that eventually lead to cellular necrosis or apoptosis (Panzarini et al., 2018). To combat the issue of toxicity against healthy cells, researchers have synthesized nanocomposites consisting of either silver-coated gold or gold-coated colloidal particles. These gold-silver nanocomposites not only reduce the toxicity exhibited by silver nanoparticles, but they also feature an increase in plasmon resonance, greater than that of pure gold. Hence, gold-silver nanocomposites may offer a safer and enhanced theranostic option for both diagnostic imaging and drug targeting of tumour cells (Chugh et al., 2018).
On the other hand, magnetic nanoparticles are commonly applied as contrast agents for medical imaging with the use of a magnetic field (MRI). Not only do they have a long retention time in circulation along with lower levels of toxicity, they are also usually biodegradable (Ventola, 2012). Unlike other materials that may become toxic in nanoscale dimensions, lipid-based nanoparticles are biocompatible and tolerable. The PEG coating improves bioavailability, biocompatibility and prolongs circulation time. This type of nanoparticle can be designed for targeted drug delivery by surface functionalization and it increases feasibility in delivering water-insoluble drugs (Murthy, 2007). Although dendrimers possess great potential for biological applications, especially as drug delivery agents, all classes of dendrimers induce cytotoxic and haemolytic effects. This safety concern can be addressed by modifying their structures (Palmerston Mendes et al., 2017). Similarly, polymeric nanoparticles are also widely studied for drug delivery, thanks to its high flexibility in varieties and sizes, tolerability and biodegradability. For example, PLGA can be metabolised and removed from the body which is safer to use. Similar to lipid-based nanoparticles, they can also be designed for targeted delivery by surface functionalization and are also strategies for solubilising water-insoluble drugs (Murthy, 2007).
Despite the optimistic assessment of nanodrugs, clinical translation is still a big challenge. Successful animal models and nanodrugs under clinical trials are discussed in the next two sections. Animal models are prompt tools to discover the feasibility of application in humans. For nanodrugs entering the stage of clinical trials, the toxicity, pharmacodynamics and safety profiles are extensively studied. The high complexity and arguable reproducibility are the main obstacles for clinical translation and regulatory approval.
ANIMAL MODELS
In theory, in vivo models facilitate the study of glioma biology and predict the effects of nanodrugs by mimicking cellular aspects of the disease in humans. However, glioma models demand a more specific and stricter compliance to the actual phenomena as compared to other cancer, due to its genetic aberrations and mosaic microenvironment (Lenting et al., 2017). Despite the hurdle, there are many preclinical studies that have been conducted to prove the efficacy of diagnosis and treatment using nanoparticles.
Murine models are the most common preclinical glioma models, and some exotic models such as zebrafish and fruit fly have also been exploited by scientists. The murine models often refer to carcinogen-induced, xenografted, and transgenic mouse or rat models, which have different features towards heterogeneity, immunocompetency and relevance of brain microenvironment. In a recent study, it was found that WK1 mouse models replicate the heterogeneity and functional characteristics of the BBB more accurately as compared to the ubiquitously utilised U87 mouse model for recurrent high-grade glioma (Brighi et al., 2020). With a more clinically applicable model, the clinical translation could be potentially fostered. The fast-growing zebrafish models cost less time and labour, hence it is useful for semi-high-throughput drug screening (Vittori et al., 2015; Welker et al., 2016); while fruit fly is deemed as a noticeably versatile model to investigate genetic aberrations. Dogs with naturally occurring glioma are also studied as a closer reference to humans (Lenting et al., 2017). Preclinical trials involving the use of passive and active targeting nanoparticles for drug delivery will be the focus in this section. Examples of successful trials applied in diagnosis will also be delineated.
Passive Targeting Nanoparticles
Passive targeting nanoparticles stabilize the drug in the bloodstream. The function of TMZ, one of the most widely employed drugs to treat brain tumours, is hindered by pH-dependent chemo-degradation at the physiological pH. Anti-cancer activity of TMZ was found to be enhanced with the use of NLC (Z. Chen et al., 2016), as well as PLGA SLN and PLGA NLC nanoparticles (Qu et al., 2016) in U87 xenograft mice. In addition, SLN did not exhibit significant toxic effects onto the BBB, as western blot analysis of occludin and claudin-1 in the BBB cells revealed no modification upon its administration (Lockman et al., 2003). Polysorbate 80-coated PBCA and polylactic acid (PLA) have essentially improved pharmacokinetics and biodistribution of TMZ in the brain of experimental rats. Such nanoparticles have also minimized the accumulation of TMZ in highly perfused organs such as heart, kidney, liver, spleen and lungs, leading to reduced toxicity (Jain et al., 2016; Tian et al., 2011). Although small interfering RNA (siRNA) and microRNA (miRNA) are powerful therapeutic strategies, their instability has hampered wide application. Cationic SLN has enhanced PEGlated c-Met siRNA accumulation in the brain and inhibited tumour growth in U87 xenograft mice (Jin et al., 2011). Polymeric nanogels are another promising carrier which have suppressed tumour growth significantly with miR-34a that is known as an important factor in oncogenic pathway (Shatsberg et al., 2016). Nanoparticles extend the drug half-life through EPR effect and increase update across BBTB owing to P-glycoprotein (P-gp) transporter inhibition on the cell membrane. Typically, this mechanism is observed for lipid nanoparticles like liposomes and SLN in vitro and in vivo, which could be further promoted by coating with surfactants such as Briji 78 or Polysorbate 80 (Ferraris et al., 2020). Furthermore, co-delivery of two different drugs or a coupled diagnostic reagent is possible for dual functions, leading to a much more desirable drug delivery system as compared to free drug solutions.
Active Targeting Nanoparticles
Active targeting nanoparticles are functionalized by binding suitable ligands to the surface so as to target the BBB via RMT and CMT. Potent protein ligands include Angiopep-2 (ANG), Apolipoprotein E (Apo E) and lactoferrin (Lf). ANG and Apo E both bind to low-density lipoprotein (LDL) receptors which are overexpressed at the BBB and in glioma cells. Lf is to be internalized by an Lf receptor which is present on the endothelial layer of the BBB (Ferraris et al., 2020). However, there are few drawbacks of engaging Lf ligand: firstly, it is not exclusively expressed on the BBB; secondly, Lf-conjugated nanoparticles have to compete with endogenous proteins which can reduce targeting efficacy and could also result in immunogenic response; and thirdly, these engineered nanoparticles might interfere with the iron homeostasis maintained by Lf receptors and evoke safety concern (Elzoghby et al., 2020). Small molecule ligands can also be incorporated to direct the nanoparticles to the BBB through glutathione (GSH) receptor, folate receptor, and monocarboxylic acid transporter (MCT-1). More ligands that target the glioma or the BBTB include EGFR, integrin, F3 peptide, matrix metalloproteinase (MMP) and interleukin (Ferraris et al., 2020).
Applications of Various Nanoparticles
ANG has been attached on polymeric nanoparticles (Zong et al., 2019), dendrimers (S. Huang et al., 2011) and PCL nanoparticles (Lu et al., 2017) to load TMZ, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) DNA and DOX respectively. Their C6 mouse models have suggested prolonged survival and higher brain uptake of the drug. Furthermore, these engineered nanoparticles exhibited biocompatibility with no noticeable tissue toxicity in mice. In another study, ATF5 siRNA has been packed into Apo E attached calcium phosphate nanoparticles and the C6 xenograft mice were shown to have an increased survival rate. Blood chemistry tests and histological analysis of the mice have also demonstrated good biosafety (J. L. Huang et al., 2017). Furthermore, saporin was loaded into Apo E installed polymersomes.
There was efficient BBB penetration and accumulation in the glioblastoma of U87 xenograft mice without observable side effects (Jiang et al., 2018). Delivering DOX in Lf bound bovine serum albumin (BSA) nanoparticles (Su et al., 2014) and cationic liposomes (Chen et al., 2011) have attained higher accumulation in the brain of C6/Wistar rats. The experiment has proven to show a longer survival time. Many other in vivo achievements have been positively displayed by designing various active targeting nanoparticles (C Ferraris, R Cavalli, P P Panciani, L Battaglia, 2020).
Moreover, the application of nanoparticles has prospered in the field of diagnosis and imaging of brain tumours is ascribable to their optical, magnetic and photodynamic characteristics. Currently, relevant animal models of in vivo imaging have been focusing on the accumulation of these nanoparticles in tumour tissue of mice via passive or active targeting (Zhang et al., 2019) . One of the studies utilizing gold nanoparticles reported a detailed high-resolution imaging of glioma cells in U87 mice brains with use of nanoscale 3D X-ray microscopy and fluorescence microscopy. In addition, there was no interference of cell viability observed. This experiment has also revealed a potential capability to monitor the subsequent development of glioma by detecting nanoparticle leakage in the induced microvasculature (Lai et al., 2015) . Another study suggested that the long-wavelength emission of red fluorescent carbonized polymeric nanoparticles is capable of achieving high photostability to detect glioma in C6 glioma-bearing rats in vivo and ex vivo, using fluorescence imaging. Such emission eliminated autofluorescence from the skull and scalp, and the tumour boundary could be clearly delineated as well. In addition, its use did not reveal significant cytotoxicity or bio-incompatibility (Liu et al., 2018) . Another research investigated ANG-conjugated PEGlyated ultra-small SPIONs which generated clear contrast enhancement of glioblastoma cells in mouse xenografts using MRI (Shevtsov and Multhoff, 2016).
CLINICAL TRIALS AND APPLICATION
While there was good preclinical evidence convincing the efficacy of nanoparticles in mouse models with intracranial tumours, clinical evidence on the application of nanoparticle based drug delivery systems is yet to be studied further. Currently, all of the clinical trials are in phase I or II which include the 10 nanodrugs being investigated for GBM (Zottel et al., 2019). Examples are nanoliposomal CPT-11, nanoliposomal irinotecan, Spherical Nucleic Acid (SNA) gold nanoparticle to target Bcl2L12 tumour gene, and PEGylated liposomal DOX. They illustrate the variety of theranostic approaches of nanoparticles (Mendes et al., 2018).
Few FDA-approved intravenous nanoparticle therapies to treat other cancers have been investigated for treatment of brain tumour and brain metastases from these cancers (Anselmo and Mitragotri, 2016). Marketed nanodrugs Marqibo® (vincristine), Onivyde MM-398® (irinotecan), Doxil® (doxorubicin) and DaunoXome® (daunorubicin) have been studied in the treatment of breast cancer brain metastases (BCBM). While liposomal formulations designed for brain metastases have yet to be approved, glutathione PEGylated liposomal DOX and liposomal cytarabine have completed clinical phase II and further assessments are underway (Shah et al., 2018).
Apart from nanoparticle drug delivery systems, there are nanoparticles that have already been implemented in clinical use. MagForce Nanotechnologies from Germany has developed a magnetic hyperthermia therapy (MHT) system called “NanoTherm®”. NanoTherm® utilizes aminosilane-coated SPIONs to generate heat and ablate the tumour cells when exposed to alternating magnetic fields (Verma et al., 2014). MHT is advantageous because besides being non-invasive, it is able to induce thermal ablation of tumour cells not only at the brain, but at any specific site of the body as well. European Medicines Agency (EMA) has approved NanoTherm® as a treatment of primary or recurrent GBM in 2013 (Gobbo et al., 2015). The clinical trial conducted by Maier-Hauff et al. in 2011 suggested extended survival time for around 7 months longer by combining MHT with fractionated RT (Maier-Hauff et al., 2011). No adverse side effects were reported. However, it is required to remove all metal implants (i.e. dental fillings) within 40 cm of the treated area and the permanent exclusion of MRI for subsequent examination owing to the MRI artifacts induced by the magnetic nanoparticles. Another clinical trial managed by Grauer et al. in 2019 with a small group of patients has further demonstrated the survival benefits of MHT treatment with RT (Grauer et al., 2019). In addition, there was potential MHT-induced anti-cancer immunological response detected, despite the development of oedema around the deposited nanoparticles.
DISCUSSION
Nanotechnology has achieved significant advancements in the past decades. Nevertheless, the development of novel tumour treatment remains in high demand as the 5-year survival rate of GBM patients is still pessimistic. With ground-breaking benefits of nanoparticles, it is possible to produce nanodrugs which are more specific and selective in detecting and treating brain tumours. Although there are only few successful clinical translations, numerous promising preclinical trials have established a sturdy foundation for further research. With regards to the limitations, safety profiles and toxicity of nanoparticles would still require more comprehensive investigation.
The heterogeneity and highly infiltrative nature of tumour cells are the main contributing factors to the poor outcome of conventional treatment. Nanoparticles can be directed to the brain tumour by passive, active and stimuli-responsive targeting. Passive targeting increases drug availability in the inter-tumoural space but its mechanism depends on the integrity of the barrier, which may be compromised in some cases. Active targeting utilises conjugated ligands on the nanoparticle which leads to selective recognition by overexpressed receptors in the tumour cells. The development of such nanoparticles must be built on the well-studied hallmarks of abnormal tissues. Stimuli-responsive nanoparticles target pathological sites via the change of stimuli like pH or temperature and this strategy has an advantage because it targets specific pathological sites. Nanodrugs are expected to alleviate the current brain tumour treatments with the aim of improving tumour targeting specificity as well as reducing the invasiveness of conventional surgical methods. Future experiments may further investigate alternative ligands to reduce the binding competition between ligand-conjugated nanoparticles and its endogenous proteins for receptor uptake. For example, an anti-transferrin-conjugated nanoparticle which binds to a different epitope as compared to the endogenous transferrin on the transferrin receptor, has reduced binding competition and thus enhanced cellular uptake and the effectiveness of the treatment (Mahmoud et al., 2020).
The use of nanoparticles has been fuelled by the need for solutions in crossing the BBB. While properties such as specific targeting, non-invasiveness and biodegradability account for the major benefits of nanoparticles as drug delivery systems, their limitations continue to impede clinical success rates and should not be overlooked. The efficacy of nanoparticles in tumour treatments are subjected to its actual size and structure even if they are able to penetrate the BBB. While some large liposomal vesicles are able to penetrate the BBB for tumour internalization, those that are too small may cross the BBB unrestrictedly and result in a risk of uncontrolled embolism (Steiniger et al., 2004). The uptake of nanoparticles by the reticuloendothelial system is also dependent on the size for each nanomaterial, which may result in subjective treatment efficacy due to the uptake and clearance of nanoparticles. This may not be easily overcome as larger nanoparticles are cleared by macrophages while smaller nanoparticles are cleared by non-phagocytic cells, resulting in uncertain rates of nanoparticle clearance (Hoshyar et al., 2016). Moreover, with dimensions similar to that of healthy living cells, the introduction of nanoparticles into the body might interfere with vital cellular processes (Teleanu et al., 2018b). In addition, the increase in surface area may result in the fusion of nanoparticles before and after penetration of the BBB. The drug dosage administered to patients could vary distinctly in the body and may result in irreversible toxicity if therapeutic drug levels are not strictly monitored (Alkilany et al., 2012). Hence, it is important to obtain the ideal particle size for effective treatment.
Neurotoxicity is another major obstacle impeding the success of nanotechnology for brain tumour treatments. Neurotoxicity is mainly caused by oxidative stress induced from the generation of ROS. Subsequent cytokine production results in inflammation and ultimately apoptosis of neuronal cells (Teleanu et al., 2018b). Aside from neurotoxicity effects, the application of nanoparticles may result in deplorable permanent alterations to the BBB, leading to fatal consequences such as brain tissue oedema or providing CNS access to toxins and molecules that are usually hindered (Kumar et al., 2017). In order to aid translational research for the improvement of brain tumour treatments, it is imperative that the benefits and adverse impacts of nanodrugs are both clearly understood.
As the first generation of nanodrugs, liposomal applications have not been concluded with significant toxicity, but the long-term health effects of nanoparticles are still unknown. While organic polymeric materials are utilised for controlled release and specific targeting, their degradation process is associated with potential toxicity (Teleanu et al., 2019). Liposomes can deliver therapeutic or diagnostic agents to the brain, but increased neurotoxicity was shown when they are used in combination with anti-cancer drug cisplatin. Inorganic nanoparticles such as gold, silver and silica are capable nanomaterials in brain tumour treatment but may gradually accumulate in the brain and develop functional impairments due to limited excretion. Hence, most nanomaterials have shown at least a minimum level of neurotoxicity, which can be taken into account in future research of nanoparticle modifications for safer applications.
Furthermore, the diverse mechanisms of administration such as inhalation, skin absorption and injection, also raises environmental health concerns especially in the manufacturing process. The development of standardized toxicological studies is essential for safer brain applications. Hence, the pharmacokinetic, drug distribution and safety profiles for each nanocarrier should be well investigated to prevent repercussions from negative side effects such as permanent alterations of the BBB and potential neurotoxicity. Further studies on brain applications may however explore new routes of administration, such as intranasal injection of anti-GBM drugs. Although it is still limited by nasal cavity restrictions and low bioavailability of peptides, it bypasses the BBB and provides a promising alternative for easing drug delivery (Hsu et al., 2021).
Other types of nanoparticles can also be exploited in the future to advance drug delivery systems. Non-spherical nanoparticles such as discoidal particles and filamentous particles seem to surpass spherical nanoparticles (such as liposomes) in terms of their cellular behaviour in the body. Biomimetic nanoparticles are an emerging class of nanoparticles which are safer as they acquire synthetic biological structures which are physiologically analogous in the human body. It is conceivable to adopt viral vectors as well due to their capability to infect cells (Taiarol et al., 2020). In light of the versatility nanoparticles possess, they could be incorporated with GBM immunotherapy, where the immunosuppressive nature of GBM is tackled. Some studies have already demonstrated that nanoparticles can be engineered to improve T-cell therapy, antigen-capturing ability and immune photothermal therapy (Samec et al., 2020). As adopting a single strategy usually hinders or increases the time taken to achieve satisfactory therapeutic effects, the integration of various nanotechnologies and molecular mechanisms can provide positive progress and direction for better management of brain tumours.
Despite its limitations, nanotechnology is gearing towards a major breakthrough in improving both clinical therapeutics and diagnostic methods for brain tumour due to benefits such as lower dosage, improved treatment efficacy, less adverse side effects, ease of drug administration with patient compliance and ultimately, an improved quality of life.
CONFLICTS OF INTEREST/DISCLOSURE
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This work was completed under the supervision of Dr. Alexis Chon and Dr. Sabrina Heng Cher Hui. Authors would like to thank Devananthini Kanniah and Tan Chao Hao Erwin, from La Trobe University, for contributing to the first draft of this manuscript.
REFERENCES
Alkilany, A.M., Thompson, L.B., Boulos, S.P., Sisco, P.N. and Murphy, C.J. (2012) 'Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions', Advanced Drug Delivery Reviews, 64(2), 190-199, available: http://dx.doi.org/https://doi.org/10.1016/j.addr.2011.03.005.
Alphandéry, E. (2018) 'Glioblastoma Treatments: An Account of Recent Industrial Developments', Frontiers in Pharmacology, 9(879), available: http://dx.doi.org/10.3389/fphar.2018.00879.
Anselmo, A.C. and Mitragotri, S. (2016) 'Nanoparticles in the clinic', Bioeng Transl Med, 1(1), 10-29, available: http://dx.doi.org/10.1002/btm2.10003.
Ashby, L.S., Smith, K.A. and Stea, B. (2016) 'Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review', World J Surg Oncol, 14(1), 225, available: http://dx.doi.org/10.1186/s12957-016-0975-5.
Becker, K.P. and Baehring, J.M. (2017) 'Current Standard Treatment Options for Malignant Glioma' in Moliterno Gunel, J., Piepmeier, J. M. and Baehring, J. M., eds., Malignant Brain Tumors : State-of-the-Art Treatment, Cham: Springer International Publishing, 123-131.
Bhaskar, S., Tian, F., Stoeger, T., Kreyling, W., de la Fuente, J.M., Grazú, V., Borm, P., Estrada, G., Ntziachristos, V. and Razansky, D. (2010) 'Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging', Part Fibre Toxicol, 7, 3, available: http://dx.doi.org/10.1186/1743-8977-7-3.
Bibhash, C.M., Narahari, N.P., Vijayaraj, S., Subas, C.D., Jayaraman, R., Jyotirmoy, D. and Biswa, M.S. (2019) 'Lipid Based Nanoparticles: Current Strategies for Brain Tumor Targeting', Current Nanomaterials, 4(2), 84-100, available: http://dx.doi.org/http://dx.doi.org/10.2174/2405461504666190510121911.
Brighi, C., Reid, L., Genovesi, L.A., Kojic, M., Millar, A., Bruce, Z., White, A.L., Day, B.W., Rose, S., Whittaker, A.K. and Puttick, S. (2020) 'Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: the importance of reproducing blood-brain barrier heterogeneity', Theranostics, 10(14), 6361-6371, available: http://dx.doi.org/10.7150/thno.46468.
Burdușel, A.C., Gherasim, O., Grumezescu, A.M., Mogoantă, L., Ficai, A. and Andronescu, E. (2018) 'Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview', Nanomaterials (Basel), 8(9), available: http://dx.doi.org/10.3390/nano8090681.
Carlsson, S.K., Brothers, S.P. and Wahlestedt, C. (2014) 'Emerging treatment strategies for glioblastoma multiforme', EMBO Mol Med, 6(11), 1359-70, available: http://dx.doi.org/10.15252/emmm.201302627.
Chen, H., Qin, Y., Zhang, Q., Jiang, W., Tang, L., Liu, J. and He, Q. (2011) 'Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas', Eur J Pharm Sci, 44(1-2), 164-73, available: http://dx.doi.org/10.1016/j.ejps.2011.07.007.
Chenthamara, D., Subramaniam, S., Ramakrishnan, S.G., Krishnaswamy, S., Essa, M.M., Lin, F.H. and Qoronfleh, M.W. (2019) 'Therapeutic efficacy of nanoparticles and routes of administration', Biomater Res, 23, 20, available: http://dx.doi.org/10.1186/s40824-019-0166-x.
Chen, Z., Lai, X., Song, S., Zhu, X. and Zhu, J. (2016) 'Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy', Drug Delivery, 23(4), 1369-1373, available: http://dx.doi.org/10.3109/10717544.2015.1038857.
Chugh, H., Sood, D., Chandra, I., Tomar, V., Dhawan, G. and Chandra, R. (2018) 'Role of gold and silver nanoparticles in cancer nano-medicine', Artif Cells Nanomed Biotechnol, 46(sup1), 1210-1220, available: http://dx.doi.org/10.1080/21691401.2018.1449118.
Clemons, T.D., Singh, R., Sorolla, A., Chaudhari, N., Hubbard, A. and Iyer, K.S. (2018) 'Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy', Langmuir, 34(50), 15343-15349, available: http://dx.doi.org/10.1021/acs.langmuir.8b02946.
Cui, Y., Xu, Q., Chow, P.K.-H., Wang, D. and Wang, C.-H. (2013) 'Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment', Biomaterials, 34(33), 8511-8520, available: http://dx.doi.org/https://doi.org/10.1016/j.biomaterials.2013.07.075.
Danhier, F. (2016) 'To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?', Journal of Controlled Release, 244, 108-121, available: http://dx.doi.org/https://doi.org/10.1016/j.jconrel.2016.11.015.
De Matteis, V., Cascione, M., Toma, C.C. and Leporatti, S. (2018) 'Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease', Nanomaterials (Basel), 8(5), available: http://dx.doi.org/10.3390/nano8050319.
Dixit, S., Novak, T., Miller, K., Zhu, Y., Kenney, M.E. and Broome, A.M. (2015) 'Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors', Nanoscale, 7(5), 1782-90, available: http://dx.doi.org/10.1039/c4nr04853a.
Duan, Y., Dhar, A., Patel, C., Khimani, M., Neogi, S., Sharma, P., Siva Kumar, N. and Vekariya, R.L. (2020) 'A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems', RSC Advances, 10(45), 26777-26791, available: http://dx.doi.org/10.1039/D0RA03491F.
Elzoghby, A.O., Abdelmoneem, M.A., Hassanin, I.A., Abd Elwakil, M.M., Elnaggar, M.A., Mokhtar, S., Fang, J.Y. and Elkhodairy, K.A. (2020) 'Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand', Biomaterials, 263, 120355, available: http://dx.doi.org/10.1016/j.biomaterials.2020.120355.
Farooq, M.U., Novosad, V., Rozhkova, E.A., Wali, H., Ali, A., Fateh, A.A., Neogi, P.B., Neogi, A. and Wang, Z. (2018) 'Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells', Scientific Reports, 8(1), 2907, available: http://dx.doi.org/10.1038/s41598-018-21331-y.
Fernandes, C., Costa, A., Osorio, L., Lago, R.C., Linhares, P., Carvalho, B. and Caeiro, C. (2017) 'Current Standards of Care in Glioblastoma Therapy' in De Vleeschouwer, S., ed., Glioblastoma, Brisbane (AU).
Ferraris, C., Cavalli, R., Panciani, P.P. and Battaglia, L. (2020) 'Overcoming the Blood-Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours', Int J Nanomedicine, 15, 2999-3022, available: http://dx.doi.org/10.2147/ijn.S231479.
Feynman, R.P. (1960) 'There's plenty of room at the bottom. An invitation to enter a new field of physics.', Engineering and Science (Caltech), 23, 22-36.
Gao, H. (2017) 'Perspectives on Dual Targeting Delivery Systems for Brain Tumors', J Neuroimmune Pharmacol, 12(1), 6-16, available: http://dx.doi.org/10.1007/s11481-016-9687-4.
García-Pinel, B., Porras-Alcalá, C., Ortega-Rodríguez, A., Sarabia, F., Prados, J., Melguizo, C. and López-Romero, J.M. (2019) 'Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment', Nanomaterials (Basel), 9(4), available: http://dx.doi.org/10.3390/nano9040638.
Gerber, A., Bundschuh, M., Klingelhofer, D. and Groneberg, D.A. (2013) 'Gold nanoparticles: recent aspects for human toxicology', J Occup Med Toxicol, 8(1), 32, available: http://dx.doi.org/10.1186/1745-6673-8-32.
Ghasemiyeh, P. and Mohammadi-Samani, S. (2018) 'Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages', Res Pharm Sci, 13(4), 288-303, available: http://dx.doi.org/10.4103/1735-5362.235156.
Glaser, T., Han, I., Wu, L. and Zeng, X. (2017) 'Targeted Nanotechnology in Glioblastoma Multiforme', Front Pharmacol, 8, 166, available: http://dx.doi.org/10.3389/fphar.2017.00166.
Gobbo, O.L., Sjaastad, K., Radomski, M.W., Volkov, Y. and Prina-Mello, A. (2015) 'Magnetic Nanoparticles in Cancer Theranostics', Theranostics, 5(11), 1249-63, available: http://dx.doi.org/10.7150/thno.11544.
Grauer, O., Jaber, M., Hess, K., Weckesser, M., Schwindt, W., Maring, S., Wölfer, J. and Stummer, W. (2019) 'Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients', J Neurooncol, 141(1), 83-94, available: http://dx.doi.org/10.1007/s11060-018-03005-x.
Hainfeld, J., Smilowitz, H., O’Connor, M., Dilmanian, F. and Slatkin, D. (2013). Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine, 8(10), 1601-1609.
Harder, B.G., Blomquist, M.R., Wang, J., Kim, A.J., Woodworth, G.F., Winkles, J.A., Loftus, J.C. and Tran, N.L. (2018) 'Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma', Front Oncol, 8, 462, available: http://dx.doi.org/10.3389/fonc.2018.00462.
Hatanpaa, K.J., Burma, S., Zhao, D. and Habib, A.A. (2010) 'Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance', Neoplasia, 12(9), 675-84, available: http://dx.doi.org/10.1593/neo.10688.
Hemmings, B.A. and Restuccia, D.F. (2012) 'PI3K-PKB/Akt pathway', Cold Spring Harb Perspect Biol, 4(9), a011189, available: http://dx.doi.org/10.1101/cshperspect.a011189.
He, Q., Liu, J., Liang, J., Liu, X., Li, W., Liu, Z., Ding, Z. and Tuo, D. (2018) 'Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective', Cells, 7(4), available: http://dx.doi.org/10.3390/cells7040024.
Hervé, F., Ghinea, N. and Scherrmann, J.M. (2008) 'CNS delivery via adsorptive transcytosis', Aaps j, 10(3), 455-72, available: http://dx.doi.org/10.1208/s12248-008-9055-2.
Hoshyar, N., Gray, S., Han, H. and Bao, G. (2016) 'The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction', Nanomedicine (Lond), 11(6), 673-92, available: http://dx.doi.org/10.2217/nnm.16.5.
Hsin, Y.H., Chen, C.F., Huang, S., Shih, T.S., Lai, P.S. and Chueh, P.J. (2008) 'The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells', Toxicol Lett, 179(3), 130-9, available: http://dx.doi.org/10.1016/j.toxlet.2008.04.015.
Hsu, J.F., Chu, S.M., Liao, C.C., Wang, C.J., Wang, Y.S., Lai, M.Y., Wang, H.C., Huang, H.R. and Tsai, M.H. (2021) 'Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update', Cancers (Basel), 13(2), available: http://dx.doi.org/10.3390/cancers13020195.
Huang, J.L., Jiang, G., Song, Q.X., Gu, X., Hu, M., Wang, X.L., Song, H.H., Chen, L.P., Lin, Y.Y., Jiang, D., Chen, J., Feng, J.F., Qiu, Y.M., Jiang, J.Y., Jiang, X.G., Chen, H.Z. and Gao, X.L. (2017) 'Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis', Nat Commun, 8, 15144, available: http://dx.doi.org/10.1038/ncomms15144.
Huang, S., Li, J., Han, L., Liu, S., Ma, H., Huang, R. and Jiang, C. (2011) 'Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma', Biomaterials, 32(28), 6832-8, available: http://dx.doi.org/10.1016/j.biomaterials.2011.05.064.
Iacob, G. and Dinca, E.B. (2009) 'Current data and strategy in glioblastoma multiforme', J Med Life, 2(4), 386-93.
Jain, D., Bajaj, A., Athawale, R., Shrikhande, S., Goel, P.N., Nikam, Y., Gude, R., Patil, S. and Prashant Raut, P. (2016) 'Surface-coated PLA nanoparticles loaded with temozolomide for improved brain deposition and potential treatment of gliomas: development, characterization and in vivo studies', Drug Delivery, 23(3), 989-1006, available: http://dx.doi.org/10.3109/10717544.2014.926574.
Jeevanandam, J., Barhoum, A., Chan, Y.S., Dufresne, A. and Danquah, M.K. (2018) 'Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations', Beilstein J Nanotechnol, 9, 1050-1074, available: http://dx.doi.org/10.3762/bjnano.9.98.
Jiang, Y., Lv, L., Shi, H., Hua, Y., Lv, W., Wang, X., Xin, H. and Xu, Q. (2016) 'PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma', Colloids Surf B Biointerfaces, 147, 242-249, available: http://dx.doi.org/10.1016/j.colsurfb.2016.08.002.
Jiang, Y., Zhang, J., Meng, F. and Zhong, Z. (2018) 'Apolipoprotein E Peptide-Directed Chimeric Polymersomes Mediate an Ultrahigh-Efficiency Targeted Protein Therapy for Glioblastoma', ACS Nano, 12(11), 11070-11079, available: http://dx.doi.org/10.1021/acsnano.8b05265.
Jin, J., Bae, K.H., Yang, H., Lee, S.J., Kim, H., Kim, Y., Joo, K.M., Seo, S.W., Park, T.G. and Nam, D.H. (2011) 'In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles', Bioconjug Chem, 22(12), 2568-72, available: http://dx.doi.org/10.1021/bc200406n.
Kaul, S., Gulati, N., Verma, D., Mukherjee, S. and Nagaich, U. (2018) 'Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances', J Pharm (Cairo), 2018, 3420204, available: http://dx.doi.org/10.1155/2018/3420204.
Kim, S.S., Rait, A., Kim, E., Pirollo, K.F., Nishida, M., Farkas, N., Dagata, J.A. and Chang, E.H. (2014) 'A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival', ACS Nano, 8(6), 5494-514, available: http://dx.doi.org/10.1021/nn5014484.
Kumar, A., Tan, A., Wong, J., Spagnoli, J.C., Lam, J., Blevins, B.D., G, N., Thorne, L., Ashkan, K., Xie, J. and Liu, H. (2017) 'Nanotechnology for Neuroscience: Promising Approaches for Diagnostics, Therapeutics and Brain Activity Mapping', Adv Funct Mater, 27(39), available: http://dx.doi.org/10.1002/adfm.201700489.
Kuramitsu, S., Motomura, K., Natsume, A. and Wakabayashi, T. (2015) 'Double-edged Sword in the Placement of Carmustine (BCNU) Wafers along the Eloquent Area: A Case Report', NMC Case Rep J, 2(1), 40-45, available: http://dx.doi.org/10.2176/nmccrj.2014-0025.
Lai, S.F., Ko, B.H., Chien, C.C., Chang, C.J., Yang, S.M., Chen, H.H., Petibois, C., Hueng, D.Y., Ka, S.M., Chen, A., Margaritondo, G. and Hwu, Y. (2015) 'Gold nanoparticles as multimodality imaging agents for brain gliomas', J Nanobiotechnology, 13, 85, available: http://dx.doi.org/10.1186/s12951-015-0140-2.
Lenting, K., Verhaak, R., Ter Laan, M., Wesseling, P. and Leenders, W. (2017) 'Glioma: experimental models and reality', Acta Neuropathol, 133(2), 263-282, available: http://dx.doi.org/10.1007/s00401-017-1671-4.
Liu, P., Huang, Z., Chen, Z., Xu, R., Wu, H., Zang, F., Wang, C. and Gu, N. (2013) 'Silver nanoparticles: a novel radiation sensitizer for glioma?', Nanoscale, 5(23), 11829-36, available: http://dx.doi.org/10.1039/c3nr01351k.
Liu, Y., Liu, J., Zhang, J., Li, X., Lin, F., Zhou, N., Yang, B. and Lu, L. (2018) 'Noninvasive Brain Tumor Imaging Using Red Emissive Carbonized Polymer Dots across the Blood-Brain Barrier', ACS Omega, 3(7), 7888-7896, available: http://dx.doi.org/10.1021/acsomega.8b01169.
Lockman, P.R., Koziara, J., Roder, K.E., Paulson, J., Abbruscato, T.J., Mumper, R.J. and Allen, D.D. (2003) 'In vivo and in vitro assessment of baseline blood-brain barrier parameters in the presence of novel nanoparticles', Pharm Res, 20(5), 705-13, available: http://dx.doi.org/10.1023/a:1023492015851.
Longmire, M., Choyke, P.L. and Kobayashi, H. (2008) 'Dendrimer-based contrast agents for molecular imaging', Curr Top Med Chem, 8(14), 1180-6, available: http://dx.doi.org/10.2174/156802608785849021.
Lu, F., Pang, Z., Zhao, J., Jin, K., Li, H., Pang, Q., Zhang, L. and Pang, Z. (2017) 'Angiopep-2-conjugated poly(ethylene glycol)-co- poly(ε-caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats', Int J Nanomedicine, 12, 2117-2127, available: http://dx.doi.org/10.2147/ijn.S123422.
Maeda, H. and Matsumura, Y. (1989) 'Tumoritropic and lymphotropic principles of macromolecular drugs', Crit Rev Ther Drug Carrier Syst, 6(3), 193-210.
Mahmoud, B.S., AlAmri, A.H. and McConville, C. (2020) 'Polymeric Nanoparticles for the Treatment of Malignant Gliomas', Cancers (Basel), 12(1), available: http://dx.doi.org/10.3390/cancers12010175.
Maier-Hauff, K., Ulrich, F., Nestler, D., Niehoff, H., Wust, P., Thiesen, B., Orawa, H., Budach, V. and Jordan, A. (2011) 'Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme', J Neurooncol, 103(2), 317-24, available: http://dx.doi.org/10.1007/s11060-010-0389-0.
Mangraviti, A., Gullotti, D., Tyler, B. and Brem, H. (2016) 'Nanobiotechnology-based delivery strategies: New frontiers in brain tumor targeted therapies', Journal of Controlled Release, 240, 443-453, available: http://dx.doi.org/https://doi.org/10.1016/j.jconrel.2016.03.031.
Matsumura, Y. and Maeda, H. (1986) 'A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs', Cancer Res, 46(12 Pt 1), 6387-92.
Mendes, M., Sousa, J.J., Pais, A. and Vitorino, C. (2018) 'Targeted Theranostic Nanoparticles for Brain Tumor Treatment', Pharmaceutics, 10(4), available: http://dx.doi.org/10.3390/pharmaceutics10040181.
Meyers, J.D., Doane, T., Burda, C. and Basilion, J.P. (2013) 'Nanoparticles for imaging and treating brain cancer', Nanomedicine (Lond), 8(1), 123-43, available: http://dx.doi.org/10.2217/nnm.12.185.
Mukhtar, M., Bilal, M., Rahdar, A., Barani, M., Arshad, R., Behl, T., Brisc, C., Banica, F. and Bungau, S. (2020) 'Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates', Chemosensors, 8(4), 117.
Murthy, S.K. (2007) 'Nanoparticles in modern medicine: state of the art and future challenges', Int J Nanomedicine, 2(2), 129-41.
Nam, L., Coll, C., Erthal, L.C.S., de la Torre, C., Serrano, D., Martínez-Máñez, R., Santos-Martínez, M.J. and Ruiz-Hernández, E. (2018) 'Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme', Materials (Basel), 11(5), available: http://dx.doi.org/10.3390/ma11050779.
Noriega-Luna, B., Godínez, L.A., Rodríguez, F.J., Rodríguez, A., Zaldívar-Lelo de Larrea, G., Sosa-Ferreyra, C.F., Mercado-Curiel, R.F., Manríquez, J. and Bustos, E. (2014) 'Applications of Dendrimers in Drug Delivery Agents, Diagnosis, Therapy, and Detection', Journal of Nanomaterials, 2014, 507273, available: http://dx.doi.org/10.1155/2014/507273.
Palmerston Mendes, L., Pan, J. and Torchilin, V.P. (2017) 'Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy', Molecules, 22(9), available: http://dx.doi.org/10.3390/molecules22091401.
Panzarini, E., Mariano, S., Carata, E., Mura, F., Rossi, M. and Dini, L. (2018) 'Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update', Int J Mol Sci, 19(5), available: http://dx.doi.org/10.3390/ijms19051305.
Pinel, S., Thomas, N., Boura, C. and Barberi-Heyob, M. (2019) 'Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment', Adv Drug Deliv Rev, 138, 344-357, available: http://dx.doi.org/10.1016/j.addr.2018.10.013.
Pope, W.B. and Brandal, G. (2018) 'Conventional and advanced magnetic resonance imaging in patients with high-grade glioma', Q J Nucl Med Mol Imaging, 62(3), 239-253, available: http://dx.doi.org/10.23736/s1824-4785.18.03086-8.
Qian, Z.M., Li, H., Sun, H. and Ho, K. (2002) 'Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway', Pharmacol Rev, 54(4), 561-87, available: http://dx.doi.org/10.1124/pr.54.4.561.
Quader, S., Liu, X., Chen, Y., Mi, P., Chida, T., Ishii, T., Miura, Y., Nishiyama, N., Cabral, H. and Kataoka, K. (2017) 'cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors', Journal of Controlled Release, 258, 56-66, available: http://dx.doi.org/https://doi.org/10.1016/j.jconrel.2017.04.033.
Qu, J., Zhang, L., Chen, Z., Mao, G., Gao, Z., Lai, X., Zhu, X. and Zhu, J. (2016) 'Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy?', Drug Delivery, 23(9), 3408-3416, available: http://dx.doi.org/10.1080/10717544.2016.1189465.
Šamec, N., Zottel, A., Videtič Paska, A. and Jovčevska, I. (2020) 'Nanomedicine and Immunotherapy: A Step Further towards Precision Medicine for Glioblastoma', Molecules, 25(3), available: http://dx.doi.org/10.3390/molecules25030490.
Saucier-Sawyer, J.K., Deng, Y., Seo, Y.E., Cheng, C.J., Zhang, J., Quijano, E. and Saltzman, W.M. (2015) 'Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue', J Drug Target, 23(7-8), 736-49, available: http://dx.doi.org/10.3109/1061186x.2015.1065833.
Shah, N., Mohammad, A.S., Saralkar, P., Sprowls, S.A., Vickers, S.D., John, D., Tallman, R.M., Lucke-Wold, B.P., Jarrell, K.E., Pinti, M., Nolan, R.L. and Lockman, P.R. (2018) 'Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases', Pharmacol Res, 132, 47-68, available: http://dx.doi.org/10.1016/j.phrs.2018.03.021.
Shatsberg, Z., Zhang, X., Ofek, P., Malhotra, S., Krivitsky, A., Scomparin, A., Tiram, G., Calderón, M., Haag, R. and Satchi-Fainaro, R. (2016) 'Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy', J Control Release, 239, 159-68, available: http://dx.doi.org/10.1016/j.jconrel.2016.08.029.
Shevtsov, M. and Multhoff, G. (2016) 'Recent Developments of Magnetic Nanoparticles for Theranostics of Brain Tumor', Curr Drug Metab, 17(8), 737-744, available: http://dx.doi.org/10.2174/1389200217666160607232540.
Shim, M.S. and Kwon, Y.J. (2012) 'Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications', Adv Drug Deliv Rev, 64(11), 1046-59, available: http://dx.doi.org/10.1016/j.addr.2012.01.018.
Silantyev, A.S., Falzone, L., Libra, M., Gurina, O.I., Kardashova, K.S., Nikolouzakis, T.K., Nosyrev, A.E., Sutton, C.W., Mitsias, P.D. and Tsatsakis, A. (2019) 'Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics', Cells, 8(8), available: http://dx.doi.org/10.3390/cells8080863.
Singh, P., Pandit, S., Mokkapati, V., Garg, A., Ravikumar, V. and Mijakovic, I. (2018) 'Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer', Int J Mol Sci, 19(7), available: http://dx.doi.org/10.3390/ijms19071979.
Sonali, Viswanadh, M.K., Singh, R.P., Agrawal, P., Mehata, A.K., Pawde, D.M., Narendra, Sonkar, R. and Muthu, M.S. (2018) 'Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer', Nanotheranostics, 2(1), 70-86, available: http://dx.doi.org/10.7150/ntno.21638.
Steiniger, S.C.J., Kreuter, J., Khalansky, A.S., Skidan, I.N., Bobruskin, A.I., Smirnova, Z.S., Severin, S.E., Uhl, R., Kock, M., Geiger, K.D. and Gelperina, S.E. (2004) 'Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles', International Journal of Cancer, 109(5), 759-767, available: http://dx.doi.org/10.1002/ijc.20048.
Stupp, R., Mason, W.P., van den Bent, M.J., Weller, M., Fisher, B., Taphoorn, M.J.B., Belanger, K., Brandes, A.A., Marosi, C., Bogdahn, U., Curschmann, J., Janzer, R.C., Ludwin, S.K., Gorlia, T., Allgeier, A., Lacombe, D., Cairncross, J.G., Eisenhauer, E. and Mirimanoff, R.O. (2005) 'Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma', New England Journal of Medicine, 352(10), 987-996, available: http://dx.doi.org/10.1056/NEJMoa043330.
Su, Z., Xing, L., Chen, Y., Xu, Y., Yang, F., Zhang, C., Ping, Q. and Xiao, Y. (2014) 'Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas', Mol Pharm, 11(6), 1823-34, available: http://dx.doi.org/10.1021/mp500238m.
Taiarol, L., Formicola, B., Magro, R.D., Sesana, S. and Re, F. (2020) 'An update of nanoparticle-based approaches for glioblastoma multiforme immunotherapy', Nanomedicine (Lond), 15(19), 1861-1871, available: http://dx.doi.org/10.2217/nnm-2020-0132.
Teleanu, D.M., Chircov, C., Grumezescu, A.M. and Teleanu, R.I. (2019) 'Neurotoxicity of Nanomaterials: An Up-to-Date Overview', Nanomaterials (Basel), 9(1), available: http://dx.doi.org/10.3390/nano9010096.
Teleanu, D.M., Chircov, C., Grumezescu, A.M., Volceanov, A. and Teleanu, R.I. (2018a) 'Blood-Brain Delivery Methods Using Nanotechnology', Pharmaceutics, 10(4), available: http://dx.doi.org/10.3390/pharmaceutics10040269.
Teleanu, D.M., Chircov, C., Grumezescu, A.M., Volceanov, A. and Teleanu, R.I. (2018b) 'Impact of Nanoparticles on Brain Health: An Up to Date Overview', J Clin Med, 7(12), available: http://dx.doi.org/10.3390/jcm7120490.
Thomsen, L.B., Thomsen, M.S. and Moos, T. (2015) 'Targeted drug delivery to the brain using magnetic nanoparticles', Ther Deliv, 6(10), 1145-55, available: http://dx.doi.org/10.4155/tde.15.56.
Thuenauer, R., Müller, S.K. and Römer, W. (2017) 'Pathways of protein and lipid receptor-mediated transcytosis in drug delivery', Expert Opin Drug Deliv, 14(3), 341-351, available: http://dx.doi.org/10.1080/17425247.2016.1220364.
Tian, X.H., Lin, X.N., Wei, F., Feng, W., Huang, Z.C., Wang, P., Ren, L. and Diao, Y. (2011) 'Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles', Int J Nanomedicine, 6, 445-52, available: http://dx.doi.org/10.2147/ijn.S16570.
Tzeng, S.Y. and Green, J.J. (2013) 'Therapeutic nanomedicine for brain cancer', Ther Deliv, 4(6), 687-704, available: http://dx.doi.org/10.4155/tde.13.38.
Urbańska, K., Sokołowska, J., Szmidt, M. and Sysa, P. (2014) 'Glioblastoma multiforme - an overview', Contemp Oncol (Pozn), 18(5), 307-12, available: http://dx.doi.org/10.5114/wo.2014.40559.
van Tellingen, O., Yetkin-Arik, B., de Gooijer, M.C., Wesseling, P., Wurdinger, T. and de Vries, H.E. (2015) 'Overcoming the blood–brain tumor barrier for effective glioblastoma treatment', Drug Resistance Updates, 19, 1-12, available: http://dx.doi.org/https://doi.org/10.1016/j.drup.2015.02.002.
Ventola, C.L. (2012) 'The nanomedicine revolution: part 1: emerging concepts', P t, 37(9), 512-25.
Verma, J., Lal, S. and Van Noorden, C.J. (2014) 'Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma', Int J Nanomedicine, 9, 2863-77, available: http://dx.doi.org/10.2147/ijn.S57501.
Vittori, M., Motaln, H. and Turnšek, T.L. (2015) 'The study of glioma by xenotransplantation in zebrafish early life stages', J Histochem Cytochem, 63(10), 749-61, available: http://dx.doi.org/10.1369/0022155415595670.
Wahajuddin and Arora, S. (2012) 'Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers', Int J Nanomedicine, 7, 3445-71, available: http://dx.doi.org/10.2147/ijn.S30320.
Wankhede, M., Bouras, A., Kaluzova, M. and Hadjipanayis, C.G. (2012) 'Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy', Expert Rev Clin Pharmacol, 5(2), 173-86, available: http://dx.doi.org/10.1586/ecp.12.1.
Wei, X., Chen, X., Ying, M. and Lu, W. (2014) 'Brain tumor-targeted drug delivery strategies', Acta Pharm Sin B, 4(3), 193-201, available: http://dx.doi.org/10.1016/j.apsb.2014.03.001.
Welker, A.M., Jaros, B.D., Puduvalli, V.K., Imitola, J., Kaur, B. and Beattie, C.E. (2016) 'Standardized orthotopic xenografts in zebrafish reveal glioma cell-line-specific characteristics and tumor cell heterogeneity', Dis Model Mech, 9(2), 199-210, available: http://dx.doi.org/10.1242/dmm.022921.
Xu, X., Li, J., Han, S., Tao, C., Fang, L., Sun, Y., Zhu, J., Liang, Z. and Li, F. (2016) 'A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration', European Journal of Pharmaceutical Sciences, 88, 178-190, available: http://dx.doi.org/https://doi.org/10.1016/j.ejps.2016.02.015.
Ying, X., Wen, H., Lu, W.L., Du, J., Guo, J., Tian, W., Men, Y., Zhang, Y., Li, R.J., Yang, T.Y., Shang, D.W., Lou, J.N., Zhang, L.R. and Zhang, Q. (2010) 'Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals', J Control Release, 141(2), 183-92, available: http://dx.doi.org/10.1016/j.jconrel.2009.09.020.
You, J., Zhou, J., Zhou, M., Liu, Y., Robertson, J.D., Liang, D., Van Pelt, C. and Li, C. (2014) 'Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres', Part Fibre Toxicol, 11, 26, available: http://dx.doi.org/10.1186/1743-8977-11-26.
Zhang, Y., Li, M., Gao, X., Chen, Y. and Liu, T. (2019) 'Nanotechnology in cancer diagnosis: progress, challenges and opportunities', J Hematol Oncol, 12(1), 137, available: http://dx.doi.org/10.1186/s13045-019-0833-3.
Zhao, M., Zhao, M., Fu, C., Yu, Y. and Fu, A. (2018) 'Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes', Int J Nanomedicine, 13, 1601-1610, available: http://dx.doi.org/10.2147/ijn.S157019.
Zong, Z., Hua, L., Wang, Z., Xu, H., Ye, C., Pan, B., Zhao, Z., Zhang, L., Lu, J., Mei, L.H. and Rutong, Y. (2019) 'Self-assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy', Drug Deliv, 26(1), 34-44, available: http://dx.doi.org/10.1080/10717544.2018.1534897.
Zottel, A., Videtič Paska, A. and Jovčevska, I. (2019) 'Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy', Materials (Basel), 12(10), available: http://dx.doi.org/10.3390/ma12101588.