Author: Condliffe Elizabeth
Institution: Mechanical Engineering
Date: September 2005
Kara surfaced, kicked a few strokes to the waiting boat, and passed her scuba gear up on deck. Moments later, like a triumphant fisherman just returned to shore, she was giving her account of the dive when the divemaster, Mark, surfaced. "Kara, are you o.k.? You came up too fast," he hurriedly interrupted her stories as he was climbing aboard. "Do you feel any tingling? If you feel anything out of sorts, any pins and needles, or any pain tomorrow, tell me immediately."
article_495_order_0
Mark was concerned about decompression sickness, which is more commonly known as 'the bends'. The nickname for this condition comes from the curled-up shape of those suffering from the severe pain it causes.
Contrary to the belief of many beginning scuba divers, the amount of air in a diver's tank is not the only factor limiting the length of a dive. Another limiting factor is the concentration of gases absorbed by body tissues, which restricts dives even when a diver's air supply is not exhausted. Most recreational divers breathe air, a mixture of 79% N2, 20% O2 and 1% all other gases. For these air-breathing divers, it is the nitrogen that causes decompression sickness.
There are other physiological risks as well, many of which are related to increased pressure caused by being underwater. The body is primarily composed of incompressible solid and liquid components, with only a small percentage of air-filled cavities. As pressure builds, the bodys solid and liquid systems evenly distribute the stress while retaining their volume. Internal pressure shifts to equal the pressure exerted by the body's surroundings. The heart, however, must work vigorously to produce a pressure greater than the ambient pressure in the body to circulate blood throughout the tissues.
The remaining components of the body are air-filled cavities, whose volumes are changeable. Most of these components are in the respiratory system: the trachea, bronchi, bronchioles and alveoli. Other components include sinuses and cavities in the middle ear. The tissues around these cavities are susceptible to barotrauma - damage caused by a change in the volume of gas that cannot be safely handled by the body.
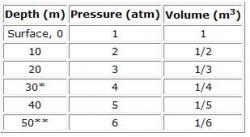
Boyle's Law helps to explain how the volume of gas in internal cavities changes while diving. Roughly, the Law states that at a constant temperature, pressure and volume are inversely proportional. The increase in pressure and corresponding decrease in volume at a certain depth of water is shown in Table 1. If a diver holds her breath, the volume of air in her lungs at the surface is halved at a 10-meter depth. Conversely, the volume of air in a diver's lungs at a 10-meter depth is doubled at the surface.
Table 1. Pressure and Volume Dependence on Depth
The lungs are especially flexible and capable of expansion. Connective tissues binding the blood vessels and airways in the lungs, in conjunction with the diaphragm, allow the lungs to expand. This allows a decrease in pressure inside the lungs, which allows an increase in volume as external pressure decreases. The inverse occurs when external pressure increases: the lungs decrease in volume as internal pressure increases. However, this system has a structural limit. If a diver ascends through the water without breathing, the pressure in the lungs could cause an expansion exceeding the exterior pressure, resulting in a lung expansion injury and burst alveoli. On the other hand, a 50-meter descent while breath-holding would decrease the volume of air in lungs that were full at the surface to below the structural minimum. The body works to prevent the rib cage from imploding by increasing pulmonary circulation, causing additional blood to collect in the lung vasculature and great veins. These pressure and volume increases help to support the ribs - but injury can still occur.
To avoid these injuries, divers equalize the pressure in their air cavities through air exchange. In the case of the respiratory cavities, divers breathe continuously during depth changes. A regulator, which is a piece of diving equipment that feeds air to the diver, provides air at ambient pressure during inhalation. Thus, a diver at 40 meters breathes air at a total pressure of 5 atmospheres (atm) (Table 1). Following Henry's Law of partial pressures and the relative gas concentrations of air, at this 40-meter depth, nitrogen is at 4 atm and oxygen is at 1 atm. Diffusion across membranes ensures that after equilibrium has been reached, the partial pressure of nitrogen in solution in the blood and tissues will also be 4 atm. The diffusion process is time-dependent, and thus most divers at 30 meters will never reach a uniform concentration of nitrogen that causes 4 atm of pressure throughout their tissues.
As pressure on a diver decreases, as in during an ascent, gases diffuse from the tissues into the blood and again from the blood to the lungs where they are expired. It is unlikely that oxygen, at its lower partial pressure, could build up to a significantly raised concentration before being used in the tissues. However, inert nitrogen can be present in high concentrations, and therefore at high pressure in the tissues. If the pressure on the diver decreases faster than the supersaturated nitrogen can diffuse out through the tissue membranes, nitrogen will form gas bubbles in the blood. These bubbles cause the physiological symptoms of decompression sickness (the bends), which range from tingling to body-curling pain. The process of gaseous bubbles leaving a supersaturated solution can most commonly be observed in soda pop, as the highly-pressurized carbon dioxide bubbles out. During Kara's quick ascent, her tissues were essentially at risk of acting like a cork in a recently shaken champagne bottle.
Whales, dolphins, seals, aquatic birds, turtles, and even sloths have all evolved better physiological systems for diving than humans have. For we humans, exploration of the undersea world for more than the duration of a single breath is only possible with technical aids, which allow our versatile bodies to be exposed to conditions for which they were not designed. Remarkably, with just a basic understanding of the underlying physiology of scuba diving, millions of people may safely explore the incredible realm beneath the surface of the water.
Suggested Reading
Albano, G. Principles and Observations on the Physiology of the Scuba Diver. Office of Naval Research, Arlington, 1970
Deakin, J. Scuba Diving. David & Charles Ltd., London, 1981.
Empleton, E. et al. (Council for National Co-operation in Aquatics) The New Science of Skin and Scuba Diving. Association Press, NY, 1967.
Edmonds, C., Lowry, C. and Pennefather, J. Diving and Subaquatic Medicine, 3rd ed. Butterworth-Heineman Ltd., Oxford, 1992