Author: Carli Bober
Institution: Clarion University of Pennsylvania
Date: April 2005
Abstract
Biofouling is the process by which living tissue or inert objects placed into aqueous environments are covered with a complex community of micro and macro organisms. The process begins with the formation of a microbial biofilm that subsequently serves as a suitable surface to which the larvae of macrofoulers attach and undergo metamorphosis into adult organisms. Biofilms can also coat medical devices imbedded in the human body and provide microbial pathogens a measure of protection from antibiotics. As such, the formation of microbial biofilms is a significant problem and strategies to inhibit their formation are needed. While several inhibitors of biofilm production have been found, a mechanism for high-throughput screening of new compounds remains largely underdeveloped. This project has sought to analyze individual steps in a dye assay proposed to measure the amount of biofilm exopolysaccharide (EPS) produced by a single marine bacterium. The dye, alcian blue, binds to half ester and carboxyl functional groups. While the use of alcian blue to quantify EPS has been previously reported, its application to measure, in situ, the amount of EPS has not been reported. To establish the efficacy of this assay and its underlying assumptions, the individual steps have been critically evaluated and their impact upon assay accuracy and reproducibility determined. We have used the microbial EPS xanthan gum as our relative standard. Results indicated that the xanthan gum standard was reproducible with a positive linear correlation (R2 = 0.9854) between change in absorbance and concentration of xanthan gum. Moreover, the residual organics and salt in seawater were found to possess a reductive effect on the absorbance of alcian blue; planktonic bacterial cells did not appear to significantly bind the dye; polystyrene plates treated with animal tissue were shown to competitively bind the dye; and that that rinsing of the biofilm did not induce loss of biofilm. Commentary is provided on the degree to which these factors must be controlled or eliminated.
Introduction
In aqueous environments the majority of bacteria grow in communities attached to surfaces (Heydorn et al. 2000; Costerton et al. 1994; Cooksey and Wigglesworth-Cooksey 1995; Characklis and Marshall 1990). They adhere to these various surfaces by producing extracellular polysaccharide (EPS) that forms a three-dimensional structure commonly termed a biofilm (Costerton et al. 1994; Cooksey and Wigglesworth-Cooksey 1995; Characklis and Marshall 1990; Cernohorska and Votava 2002). Bacterial biofilms provide advantages in harsh environments. For example, the harbored bacteria gain a measure of protection and can more easily compete for nutrients (Costerton et al. 1994). Biofilm based bacteria are less exposed to a host's immune response and less susceptible to antibiotics than planktonic cells (Cernohorska and Votava 2002). Biofilms are unique environments whereby the sessile cells are phenotypically different from their planktonic counterparts, often expressing multiple genes that would otherwise remain unexpressed during planktonic growth (Costerton 1995; Costerton et al. 2003; Bryers 2000). This density-dependent behavior is thought to be controlled by cell-cell communication pathways in which free diffusible compounds, termed autoinducers, bind to regulatory proteins and form, in the process, regulatory complexes that bind to promoters and activate target genes coding for the multicellular-like behavior associated with biofilm-based bacteria (Cernohorska and Votava 2002; Davies et al. 1998; Dunny and Winans 1999; Bassler 2002).
In the marine environment, most surfaces are susceptible to bacterial colonization and formation of microbial biofilms, an event that precedes biofouling. While biofilms are ideal environments for bacteria, they can cause damage to certain surfaces such as marine sea grass and algae (De Nys and Steinberg 1999). In this scenario, the microbial biofilm induces the settlement and metamorphosis of macrofoulers (i.e., a biofouling community) that can block light access to the plants photosynthetic cells on the fronds. The microbial films also attract predatory grazers searching for food. While researchers have been working towards anti-fouling materials to coat man made objects, some marine plants have evolved a mechanism to inhibit the growth of microbial biofilms by producing compounds that inhibit the initial act of biofilm formation. The most prominant example is the Australian red alga, Delisea pulchra, which produces a group of water insoluble secondary metabolites that inhibit the density-dependent cell-cell communication pathways that control biofilm formation (De Nys et al. 1993, 1994).
The discovery of these compounds, known as the halogenated furanones, has opened up a new class of natural products with important medical and industrial applications. To better screen for the presence of these and similar compounds in extracts of marine algae collected from Hawaiian waters, efficient mechanisms for high-throughput assessment of biofilm formation are needed. Established procedures used to assess biofilm formation include high resolution imaging techniques such as confocal laser-scanning microscopy and molecular characterizations such as fluorescence in situ hybridization. These types of techniques, however, are not amenable to high-throughput screening and are often invasive. More appropriate techniques are needed to measure the mass quantity of biofilm EPS produced. One such way is to use dyes that bind to EPS functional groups. While the use of alcian blue to measure EPS from bacteria has been previously reported, its use to measure, in situ, the amount of biofilm EPS has not been attempted. This report reviews the efficacy of an alcian blue assay proposed to quantify the amount of biofilm EPS by measuring the amount of alcian blue that binds to the EPS half-ester and carboxyl functional groups.
Methods & Materials
Preparation of Reagents
A 0.015 mg/mL solution of alcian blue was prepared in 7% acetic acid as follows. The solution was heated while stirring for 10 minutes and then continuously stirred without heat for 2 hours. The absorbance was read at 600 nm and recorded. The solution was then centrifuged at 5000 rpm for 30 minutes under refrigeration. The supernatant was collected and the absorbance read at 600 nm. The solution was then filtered through a 0.45 µm Whatman nylon filter under vacuum. The absorbance was again read and recorded at 600 nm. This process was repeated with a 0.2 µm nylon syringe filter and the absorbance read again. Finally, a small aliquot of solution was centrifuged at 10,000 rpm for 5 minutes at room temperature. The absorbance of the supernatant at 600 nm was again measured. When no change in absorbance was observed between the final two steps, it was assumed that that alcian blue solution was fully dissolved with no precipitate present. A stock solution of 0.05% xanthan gum solution was prepared in 95% ethanol and stored at 4 ºC.
Strain Selection and Maintenance
A Pseudoaltermonas luteoviolacea culture was obtained as a generous gift from Dr. Michael Hadfield. The strain, isolated from a multi-species marine biofilm, was first screened for purity by streaking on half seawater tryptone agar plates to ensure isolation of individual colonies. A pure colony was then picked and grown overnight in modified seawater-tryptone media (0.025% tryptone, 0.015% yeast extract, 0.0015% glycerol). A master seed band was created by freezing one hundred 1 ml aliquots of this solution in 20% glycerol at minus 80. To ensure purity and to reduce genetic drifting, each experiment was started from a new aliquot taken from the master seed bank.
Alcian Blue Binding Assay
12 hour Pseudoaltermonas luteoviolacea cultures were brought to a standard OD of 1.000 +/- 0.005. 2 mL aliquots of this cell suspension were transferred into individual wells of polystyrene multi-well plates and allowed to stand for 8 hours. The aqueous solutions above were then decanted and the resulting biofilms were then gently rinsed three times with 1 mL of deionized water. The films were allowed to air dry for 30 minutes before 2 mL of 7% acetic acid was added to each biofilm and the entire multiwell plate placed in an ultrasound bath for 15 minutes. A 2 mL solution of 0.015 mg/mL alcian blue was then added to each well and allowed to react for 30 minutes. A 1 mL aliquot of the supernatant was then pipetted from each well, centrifuged at 10,000 rpm, and its absorbance read at 600 nm. The amount of dye that bound to the biofilm was taken as difference between the absorbance of this supernatant and that of the control, which did not have a biofilm present. This value is reported as a delta absorbance and can be converted to concentration of EPS in terms of mg/ml by correlation to the xanthan gum standard.
Xanthan Gum Standard
Ten volumes of the xanthan gum working solution were prepared from the stock solutions in concentrations of 0, 0.150, 0.750, 1.500, 2.250, 3.000, 3.750, 4.500, 5.250, 5.625 and 6.000 μg/mL. A standard calibration curve was generated using the alcian blue assay. A typical curve is given in Figure 1.
Figure 1. Calibration curve for xanthan gum. The delta absorbance refers to the change in solution absorbance due to alcian blue binding to xanthan gum and precipitating out of solution.
Experimental
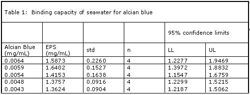
Particulate Matter in Seawater Binding Test. Five aliquots of alcian blue (0.0064, 0.0059, 0.0054, 0.0048, 0.0043 mg/mL) were made to a final volume of 1.4 ml. Each aliquot contained seawater (29% v/v) and acetic acid (7% v/v). The tubes were then vortexed for 45 seconds and allowed to stand for 15 minutes. These solutions were centrifuged at 10,000 rpm for 5 minutes, the supernatant decanted and its absorbance read at 600 nm. The control used DI water in place of the seawater.
Bacterial Cell Binding Test. Four 50 mL cultures of Pseudoaltermonas luteoviolacea were centrifuged at 4000 rpm for 10 minutes and the cells resuspended in a saline solution to achieve a final optical density of 1.00 at 600 nm. Varying concentrations of acetic acid were then added to the solution. The cell/acetic acid mixture was then vortexed for 45 seconds and let to stand for 15 minutes. Alcian blue solution was then added at 0.0120, 0.0030, 0.0008, and 0.0000 mg/mL concentrations to bring the final volume of each sample to 1.8 ml. The final concentration of acetic acid in each solution was 7%. The mixture was then vortexed for 45 seconds and let to stand for 30 minutes. The samples were then centrifuged at 10,000 rpm for 5 minutes, the supernatant decanted, and its absorbance read at 600 nm. The amount of alcian blue that bound to the surface of the cells, and thereby precipitated from solution, was taken as the difference between this absorbance and that of the control solution, which contained no cells. Surface Material of the Multiwell Plate Binding Test. 2 ml solutions of acetic acid (7%) and were added to treated and untreated polystyrene multi-well plates and let to stand for 15 minutes. A 2 mL solution of 0.015 mg/mL alcian blue was added and the mixture allowed to stand for 30 minutes before 1 mL solutions were decanted and their absorbance read at 600 nm. These values are compared relative to each other and without subtraction of a control. Rinse Binding Test. The rinse solutions from the alcian blue assay described above were collected as an aggregate solution and then divided into twelve 400 µl sub-samples. Equal volumes of 7% acetic acid was added, the solution vortexed for 45 seconds, and then let to stand for 15 minutes. A 400 µl aliquot of a 0.015 mg/mL alcian blue solution was then added to each sub-sample to yield an alcian blue final concentration of 0.0075 mg/ml and a total solution volume of 1.6 ml. The solution was then allowed to react for 30 minutes, centrifuged at 10,000 rpm for 5 minutes, and the absorbance of the supernatant measured 600 nm. The absorbance of each sub-samples are reported as a composite average with standard deviations. Two types of controls were used: (1) sterile DI water and (2) saline solution. The controls were treated identically as the rinse solutions except that DI or saline solution was used and only three replicates were averaged. Values for the rinse solution and controls are reported in units of alcian blue absorbance. The amount of alcian blue precipitated from solution can be taken as the difference in absorbance between the rinse sample and the control, whether it is the DI or saline solution. Statistics
The 95% confidence intervals were calculated as per accepted statistic principles. For each data point set, a mean (MN), its standard deviation (std), and the standard error (stderror) of the mean were calculated. Confidence limits (CL) were then obtained for 95% from tables using the appropriate degree of freedom (e.g., n 1, where n is sample number). The limits were then calculated as:
(1) MN ± CN * stderror
Results
Table 1.
The Effect of Seawater on Alcian Blue Binding. To test the hypothesis that particulate matter in seawater may competitively bind and precipitate alcian blue, the amount of alcian blue lost from solution was assayed relative to a control that contained deionized water. The results, which are shown in Table 1, indicate that despite the amount of alcian blue present, a relatively constant range of 1.4-1.6 mg/ml of alcian blue was precipitated from solution in the presence of the seawater. The four replicates resulted in a mean value (1.4762 mg/ml) within the 95% confidence interval of 1.2456 and 1.7067. Therefore, it can be stated with confidence that particulate organic carbon matter and transparent exopolymer particles are present in seawater but that the measurement has a lot of variability. Variability in confidence limits, which is not seen with assays that use DI water (data not shown), suggests that some property of the seawater possesses destabilizing effect on alcian blue precipitation. The Effect of Bacterial Cells on Alcian Blue Binding. To test the hypothesis that bacterial cells within the biofilm matrix may bind alcian blue, and thus interfere with the assay by duplicating the binding effect of biofilm half ester and carboxyl groups, the amount of alcian blue bound by planktonic cells was measured. The results, which are shown in Table 2, suggest that binding of the alcian blue by the bacterial cells was undetectable. Although slightly broad, the 95% confidence limits on each data point are sufficiently broad to suggest that the data presented is indistinguishable from zero. It is assumed that the large variance in the confidence limits has arisen from the fact that since no binding occurred, the difference when subtracting two large but nearly equal numbers may appear to yield a significant number but in fact does not.
Table 2.
The Effect of Treated and Untreated Multiwell Plates on Alcian Blue Binding. To test the hypothesis that the treated and non-treated polystyrene multiwell plates bind alcian blue, dye was placed into the wells and the amount of dye lost from solution measured. Similar measurements were also conducted on glass and polyurethane falcon centrifuge tubes. Table 3 presents the absorbance values of solutions after they were decanted from the hold vessel. The absorption of the solutions decanted from the treated and untreated polystyrene plates were essentially equivalent. By contrast, both the glass and polyurethane falcon tubes bound significant amounts of the dye. That said, their relative competitiveness for the dye cannot be commented upon because the lack of multiple replicates (only two were taken) has lead to rather high spread on our 95% confidence limits. Determination of EPS Lost in the Rinsing Step. To test the hypothesis that biofilm EPS may be lost in the rinsing step of the alcian blue assay, the rinse solution was tested for its capacity to bind alcian blue. Two rinse solutions were used: DI water and saline. The saline control was used because of previous results (Table 1) that suggested some property of the seawater increased the variability in the measurement (as measured through increased range on the 95% confidence limits). The results presented in Table 4 show that the absorbance of the DI water rinse treatment is similar to that of the DI water control, suggesting that negligible EPS was lost during the rinsing step. In contrast, the saline rinse had a much lower absorbance. Saline, as opposed to seawater, does not contain organic particulate matter or transparent exopolysaccharide particles that can form an insoluble precipitate with the alcian blue. As such, it was assumed that the presence of high salt concentration does interfere with the absorption of alcian blue at 600 nm. The confidence limits for both controls are roughly similar, therefore, there is confidence that this observation is real. Work is now underway to investigate why elevated osmolarity affects the absorption of alcian blue.
Table 3.
Discussion
The dye alcian blue has been used to measure biofilm EPS through its ability to form insoluble precipitates after binding to the side groups of biofilm EPS (Passow and Alldredge 1995). Alcian blue is a cationic copper phthalocyanine dye that complexes with anionic carboxyl and half-ester sulfate groups (Ramus 1977). Alcian blue reacts with the acidic functional groups of polysaccharides yielding an insoluble non-ionic precipitant (Passow and Alldredge 1995). This reaction is shown in Figure 2. As this dye can be read spectrophotometrically, the quantity of EPS present can be approximated by measuring the amount of dye that is removed from solution as a result of the precipitation process. The actual amount of EPS can then be approximated in terms of mg/ml by comparison against a calibration curve generated for a known biofilm EPS, such as xanthan gum, an exopolysaccharide produced by the bacterium Xanthomonas campestris (McKellar et al. 2003). Theoretically, this principle allows for the quantitative estimation of biofilm EPS.
Table 4.
If successful this method is particularly useful because its spectrophotometric basis of detection allows for the process to be automated in 96 well-plate format using robotics and micro pumping systems. This is in direct contrast to other techniques used to measure biofilm properties such as confocal laser scanning microscopy, or fluorescence in situ hybridization (FISH), which provide visual information but do not directly quantify homogeneous parameters such as the mass of EPS.
The xanthan gum standard proved to be reproducible with a positive linear correlation between the quantities of alcian blue precipitated out of solution (e.g., as measured by the delta absorbance) and the concentration of xanthan gum present. In terms of the specific application of our assay, we found that the seawater should not be used as a rinse solution, despite concerns that DI water applied to marine bacteria might lyse cells, thus releasing intracellular compounds that could bind to and form a precipitate with alcian blue. The high salt concentration was also found to possess a reductive effect on the absorption spectra of alcian blue, possibly due to the high salt concentration interfering with the cationic-anionic binding interaction between the dye and the EPS functional groups.
Figure 2. Molecular structures of anionic xanthan gum and cationic alcian blue.
The effect of surface chemistry of the materials upon which our biofilms are formed was also tested. It was found that the polystyrene plates pretreated with animal tissue, did not bind relatively more alcian blue than the untreated plates. This occurred despite our concerns that the hydroxyl groups of the treated plates would, when primed with acetic acid, could bind additional alcian blue. It is possible that the presence of the acetic acid (7%) in the absence of buffer competitively bound to the hydroxyl groups and thus minimized their interaction with the positively charged dye. By contrast, two other materials tested, glass and polyurethane, clearly bound relatively more of the alcian blue than the polystyrene plates, highlighting the need to quantify these effects or to keep them constant over controls and trials. In other tests, no statistical evidence of alcian blue binding to the cells could be statistically shown within 95% confidence.
Finally, the rinsing step of the assay did not dislodge EPS from the surface attached biofilm regardless of whether DI water or saline solution was used as the rinsing fluid. This encourages the use of DI water despite concerns that solutions of low osmolarity will lyse the cells in biofilms made from marine bacteria. This is considered advantageous in terms of removing salts that can interfere with the alcian blue-EPS precipitation step.
Acknowledgments
The authors gratefully acknowledge support from NSF grant #023600 and the University of Hawaii Sea Grant College Program.
References
Heydorn A, et al. (2000). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 146:2395-2407.
Costerton JW, et al. (1994). Biofilms, the customized microniche. J
Bacteriology. 176:2137-42.
Cooksey KE and B Wigglesworth-Cooksey. (1995). Adhesion of bacteria and diatoms to surfaces in the sea: a review. Aquatic Microbial Ecology. 9:87-96.
Characklis WG and KC Marshall. (1990). Biofilms. New York: John Wiley & Sons. 341-94.
Cernohorska L and M Votava. (2002). Biofilms and their significance in medical microbiology. Epidemiol Mikrobiol Immunology. 4:161-4.
Costerton JW. (1995). Overview of microbial biofilms. J Industrial
Microbiology. 15:137-140.
Costerton JW, et al. (2003). The application of biofilm science to the study and control of chronic bacterial infections. J Clinical Investigation. 112:1466-1477.
Bryers JD. (2000). Biofilms: an introduction, in biofilms II: process analysis and applications. J.D. Bryers, Editor. Wiley-Liss: New York. 3-13.
Davies DG, et al. (1998). The involvement of cell-cell signals in the development of a bacterial biofilm. Science. 280:295-298.
Dunny GM and SC Winans. (1999). Bacterial life: Neither lonely nor boring. Pages 1-5 in: Cell-Cell Signaling in Bacteria, in Cell-Cell Signaling in Bacteria, G.M. Dunny and S.C. Winans, Editors. 1999, American Society for Microbiology
Press: Washington, D.C. p. 1-5.
Bassler B. (2002). Small Talk: cell-to-cell communication in bacteria. Cell. 109:421-424.
De Nys R and PD Steinberg. (1999). Role of secondary metabolites from algae and seagrasses in biofouling control. Recent Advances in Marine Biotechnology. Vol III, Biofilms, Bioadhesion, Corrosion, and Biofouling. 223-244.
De Nys R, et al. (1993), New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron. 49:11213-20.
De Nys R, et al. (1995). Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling. 8:259-71.
Passow U and AL Alldredge. (1995). A dye-binding assay for the spectrophotometer measurement of transparent exploiter particles (TEP). Limnol Oceanogr. 7:1326-35.
Ramus J. (1977). Alcian blue: a quantitative aqueous assay for algal acid and sulfated polysaccharides. J Phycol. 13:345-8.
McKellar RC, et al. (2003). Influence of culture and environmental conditions on the composition of exopolysaccharide produced by Agrobacterium radiobacter. Food Hydrocolloids. 17:429-37