Author: Ghatalia Pooja
Institution: Molecular Biology
Date: April 2006
A German guided missile, the 46-foot tall, 14-ton behemoth "Vengeance," swooped thousands of miles in less than five minutes to find its vulnerable target, downtown London. Guided military rockets like these shook all in the 1940s during the Second World War.
"Mega missiles" did exactly what one would now expect a "nano micelle" to do. (Nano micelles are tiny particles made from a material that is compatible to the body and can be broken down in the body. These micelles can contain drugs.) The time wheel has spun, and now the smaller, the better. Now we talk in the scale of nanometers, which is one-billionth of a meter, or about one-millionth the size of a pin head.
The Germans wanted to wipe out the Allies, and scientists wish to obliterate cancers. What Wernher von Braun thought when he found a target-specific missile in 1940 is what the scholars in MIT and Harvard want to achieve when they send their nano micelle into the body.
In all probability, Omid Farokhzad of Harvard Medical School did not derive inspiration from the homing device of the Nazis, but his goal is to deliver the powerful cancer drugs right to those cancer cells and no other cells in the body.
"We have designed targeted drug delivery vehicles that attach to and get taken by cancer cells and kill the cancer," says Farokhzad, the principal investigator of the study.
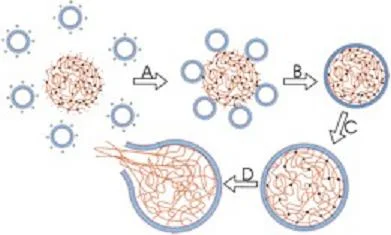
Figure 1. Nanoparticles releasing the contained drug at the exact site of tumor.
But why should we care to be target-specific when delivering drugs? One of the reasons is to prevent harmful side-effects. A difficulty of current cancer therapy is that the injected drug goes everywhere throughout the body, causing the patient to lose hair and experience severe nausea. The cancer drug is like a "free-drug," interacting with fast-replicating skin cells leading to hair loss, for example.
Besides causing these side-effects, non-targeted drug pills have to face the acidic stomach, the cell membranes, and the various cleavage enzymes that are ever-ready to break them down. The drug loses most of its efficacy before it even reaches the target site. Non-specific drugs enter the circulation at a lower rate than other drugs, and they persist in the body's tissues for a shorter duration, making them less effective. (This process by which a drug is absorbed into the blood stream is called pharmacokinetics.)
Therefore, the goal of the target drug delivery system is to deploy intact medications to specifically targeted parts of the body, to protect the drugs from breakdowns and to increase the drug absorption into the bloodstream.
To attain this goal, Farokhzad and his team developed nanoparticles (which act like cargo carriers) attached to a homing/tracking device (a highly folded RNA or DNA fragment.) Imagine a sponge soaked in water. Now shrink the sponge to a size of 50-250 nanometer (about one-tenth the diameter of human hair.) If the sponge was the nanoparticle, then the water would be the drug that the nanoparticle encloses. The RNA/DNA fragment guides the nanoparticle to the target site, and this fragment is called an "aptamer." This means that doctors could inject patients with nanoparticles that get inside tumors and release powerful doses of cancer-killing drugs while leaving the rest of the body unscathed.
Indeed, nanotechnology in the ideal world would work wonders. In the past ten years, the field has exploded into a $13 billion industry. But some, like Gwen Ruta of the Environmental Defense, suggests that the federal government should spend more money into researching the risks of nanotechnology.
"History is littered with technologies like pesticides or DTT which marvelously killed insects, but also turned out lethal to birds of prey like eagles and falcons," says Ruta. "Preliminary studies have shown that some nanomaterials are able to damage skin, brain, and lung tissue. Technological advances like these, later turn into disasters."
Farokhzad agrees that nanotechnology does have some hazards, but he says the public is protected by the existing government safeguards in the medical field and by a growing body of knowledge about nanotechnology.
"All the materials that we have used for this innovation have been used inside the human body for about 30 years and are FDA approved," says Farokhzad. "This is the birth of a super-molecular nanostructure."
While Ruta forsees accumulation of nanoparticles in body tissues and the environment, Farokhzad believes that the combination of targeted delivery and controlled release of drugs at the site of cancer will likely result in smart therapeutics' that are more effective and safer than what is available today.
According to Jinming Gao, an associate professor at University of Texas Southwestern Medical Center and an expert in cancer nanomedicine, the smart therapeutics' are much more reliable and safer than conventional drugs. By smart therapeutics,' he means engineered drugs that are target-specific.
"Instead of telling the patient here you go...try this drug,' scientists should be able to decide the best drug to kill the cancer and to distribute it correctly in the body," says Gao. "One has to be very specific in such cases where medicines have deleterious side-effects."
According to some, a controlled and monitored approach towards nanotechnology might be the best solution.
Some predict that within the next two decades, we will be able to inject these nano micelles for any disease into our bodies with the expectation that they will go to enemy cells, enter the enemy homes, and do their action there without creating any civil havoc.