Author: Laurie Sorenson
Institution: Hawaii Pacific University
Date: February 2007
Abstract
Triggerfishes (family Balistidae) are members of one of the most derived group of fishes, the Tetraodontiformes. Species of this family employ a median paired fin (MPF) swimming style that recruits the use of the dorsal and anal fins for propulsion. This study used ultra-thin slice plastination to investigate median fin myology of Rhinecanthus rectangulus, a common triggerfish within the Indo-Pacific region. This method maintained detail and accurate spatial relationships between anatomical features, allowing accurate rendering of the vertebral column, four sets of pterygiophores, one myotome, and two sets of muscle groups along the median fins. Four muscle bundles were apparent between each set of pterygiophores. The inner bilateral muscle pairs had distinct coloration suggesting they may be oxidative (red) muscle groups. Lateral bands of red muscle were also present along the length of the body, and their location was evident by the indentations in the myotome structure. The arrangement and function of muscles controlling the median fins appear to be more complex than previously described, suggesting that advanced morphological features have evolved in triggerfishes to employ MPF swimming.
Introduction
Coral reefs are relatively shallow, dynamic environments that harbor numerous marine species. Hundreds to thousands of fish species can be found within a single reef complex (Moyle & Cech 2004). It is among the irregular cracks and crevices of coral heads and rocks that these species evolved into the diverse array of reef fishes present today. Fishes that inhabit coral reefs have developed a variety of adaptations necessary for survival. One of the most derived groups that have evolved is the Tetraodontiformes. Fishes in this order may have body forms that vary greatly from the typical streamlined acanthopterygian (spiny-rayed fish) shape, and most have developed defense mechanisms for protection against predators (Moyle & Cech 2004). The triggerfish (family Balistidae) are of the Tetraodontiform order and are highly specialized for the coral reef environment.
Triggerfish have a deep, compressed body form; this shape is useful in increasing the potential for swift, precise maneuvers in tight areas around the reef, and makes triggerfish well adapted for feeding among coral heads and along the sea floor (Moyle & Cech 2004). These fishes lack pelvic fins and have enlarged dorsal and anal fins located opposite one another in the posterior region of the body. The use of the dorsal and anal fins for propulsion is one of the most notable characteristics of balistids, thus it is termed balistiform swimming.
Balistiform swimming is a type of median paired fin (MPF) locomotion. MPF swimming involves undulation of the paired (pectoral) fins located on the sides, or the median fins along the sagittal plane of the trunk, while keeping the body rigid. In contrast, body caudal fin (BCF) swimming describes typical fish locomotion and entails undulation along the entire length of the body and caudal fin. BCF swimming is argued to be more efficient and the best means of achieving maximum acceleration, turning couples and sprint speeds, whereas the evolution of the MPF swimming method is believed to allow greater agility and stability, especially at slow speeds (Webb 1982).
The dependence on median fins for locomotion has caused the dorsal and anal fins of triggerfish to enlarge and become very flexible. This flexibility is necessary for the fish to control undulatory waves that are passed along their length (Webb 1982) and produce thrust in several directions (Webb 1998). Webb (1984) proposed that using the median fins located around the posterior end of the body while holding the body rigid could minimize drag by making the anterior end more streamlined. He also compared the mean drag generated by a flexing body and a rigid body and found that rigidity can decrease drag by 2-5 times (Webb 1982). In addition, Lighthill & Blake (1990) proposed that holding the anterior region of the body rigid and undulating the median fins not only reduces drag, but also increases the momentum of water accelerated by the propulsors. Reducing drag and building momentum is advantageous because it allows achievement of higher speeds and efficiency using MPF swimming.
Fish propulsion is achieved by the recruitment of two basic types of muscle. Red muscle (slow twitch, oxidative) is used for sustained aerobic activity, and white muscle (fast twitch, glycolytic) is employed for brief bursts of anaerobic activity. In most BCF swimming fishes, superficial bands of red muscle lie laterally along the sides of the body and are used for sustained swimming (Moyle & Cech 2004). These muscle groups are present in some triggerfish; however, balistids also appear to exhibit red muscle adjacent to the median fins that control movement of the fin rays. The majority of body musculature in fishes is generally white and arranged in units called myotomes. These segments of muscle exhibit a W-shape and are arranged longitudinally along the body (Bond, 1996). Each myotome is folded into a complex arrangement that forms a series of nested cones with adjacent muscles. They are separated by collagenous sheets of tissue called myosepta, which are believed to be involved in transmitting muscle fiber forces (Van Leeuwen 1999).
The structure of muscle groups powering the median fins in Tetraodontiformes deviates from that of typical BCF swimming fishes, as described by Winterbottom (1974). Dorsal and anal fin rays in fishes are generally controlled by paired erectors and depressors that manipulate fin rays into an upright and depressed position, respectively. The erectors lie superficially to the depressors and connect the front of the pterygiophores, the bones between vertebral spines that provide support to the dorsal and anal fins, to the anterior faces of the fin ray bases. The depressors join the posterior faces of the pterygiophores to the backs of the fin ray bases. Both muscle groups are found in bilateral pairs along the sagittal plane. The dorsal and anal fins are also controlled by the inclinator muscles that are found just beneath the skin. These muscle groups are shorter and are also present in pairs flanking the sagittal plane. In most fishes inclinators manipulate the fins laterally in a side-to-side motion (Cailliet et al. 1986).
In triggerfish however, muscles controlling lateral motion must be well developed, and it appears that the erectors and depressors, as described for balistids by Winterbottom (1974), have evolved to provide this function. Both groups of muscles are arranged in bilateral pairs along the sagittal plane between pterygiophore flanges. Winterbottom (1974) described the erectors as connecting the posterior region of the pterygiophores to the anterolateral sides of the fin ray bases, and the depressors as joining the anterior area of the pterygiophores to the posterolateral sides of the fin ray bases. The depressors and erectors function as the inclinator muscles as described in typical fishes, to move the fin rays laterally, or side-to-side. Inclinator groups are also present in balistid species just under the skin. These muscle bundles are shortened and are inserted into the fin ray base laterally and may also be involved in fin swimming movements.
Ultra-thin slice plastination was used in this study to examine the three-dimensional relationship of muscle groups and skeletal features of the triggerfish. This method has proven to be successful in examining anatomy by providing transparent cross-sectional views of tissues, while maintaining the original positions of structures. Plastination was first developed in 1979 (Von Hagens 1979) and has been used since for a variety of functions, but especially in the clinical and medical fields. Previous studies have found that plastinated specimens provide the potential to investigate ailments (Sora & Genser-Strobl 2005), facilitate morphometric measurements (Genser-Strobl & Sora 2005; Lozanoff 1992; Lozanoff & Diewert 1989; Lozanoff et al. 1994), and correctly identify anatomical features (Thomas et al. 2004). The use of plastinated specimens has grown in popularity because they act as dry, non-toxic models for use in education and surgical approaches (Henry 2005; Douglass & Glover 2003; Baeres & Møller 2001; Maeta et al 2003; Grondin 1998).
Methods
A Rhinecanthus rectangulus (reef triggerfish) specimen was caught by spear offshore of Oahu, Hawaii near Makapu'u Point. The head was removed and the remaining posterior region, with median fins intact, was preserved in 10% formaldehyde solution diluted in seawater for five days. The specimen was transferred to tap water for two days, followed by five days in 40% ethanol solution. The ethanol percentage was gradually increased up to a final concentration of 70% over two days.
Ultra-thin slice plastination was used to obtain 1 mm thick slices of the specimen according to the methods described in Sora et al. (2006). The specimen was frozen at -25° C for two days, after which it was dehydrated in a series of increasing concentrations of cold (-25° C) acetone over 63 days. In the final acetone solution, the specimen was allowed to warm to room temperature (>15° C). The acetone was replaced with methylene chloride at room temperature for 28 days, which degreased the sample.
The specimen was placed in E12 impregnation mixture (Von Hagens 1986), containing a polymer, hardener and an accelerator, within a Heraeus VT6130 M vacuum drying oven (Hareaeus Instruments, Kendro Laboratory Products GmbH) set at 30° C. Impregnation of the sample began the following day by applying increasing pressure over a period of five days. On the fifth day, the temperature of the drying oven was increased from 30° C to 60° C. The specimen was placed into a mold that was filled with E12 impregnation mixture and dried at 65° C for four days. The hardened sample was finally cut into 1 mm thick sections paralleling the frontal plane using a 0.4 mm width Exact 310 CP saw (Exact Apparatebeu GmbH, Norderstedt, Germany).
The slices were scanned at a resolution of 600 dpi and were loaded into the WinSURF 4.2 program for reconstruction (Moody & Lozanoff 1998). The skin outline was contoured manually and was used as a reference to properly align each individual slice. Manual contouring was completed for a myotome along the width of the body, as well as the vertebrae, four sets of pterygiophores, and two sets of muscle groups adjacent to the dorsal and anal fins. Sets of pterygiophores located between spines of the dorsal arch were contoured as a group due to their proximity to one another. Separate files were generated for each set of features, which were checked for proper alignment and discontinuities. Aberrations were resolved either by adjusting the alignment, or using the smoothing feature in the 3D function where features were rendered together to observe spatial relationships.
Results
Ultra-thin slice plastination proved a successful method for reconstructing the R. rectangulus sample in three dimensions. This approach allowed accurate rendering of skeletal and muscular features, providing a means to examine morphological characteristics with ease. The WinSURF 4.2 program was a straightforward interface that helped to contour features efficiently and manipulate the model with little difficulty.
Figure 1: Plastinated slice (frontal sectioning: perpendicular to dorsal-ventral axis) from the posterior, hypaxial region of R. rectangulus showing relative locations of the pterygiophores, trunk muscle, and median fin muscle groups. The red muscle groups along the vertebral column show a distinct pigmentation in comparison to other muscle groups.
Figure 2: Three-dimensional rendering of skin surface (grey, transparent), vertebral column (yellow) and myotome (white) showing indentations where lateral red muscle is present. The myotome shows the W-shape typical of trunk muscle in fishes. Posterior direction is to the right and projecting into the image.
The slices maintained detail and accurate spatial relationships to other anatomical features with minimal distortion (Figure 1). These relationships were easily converted to 3D by loading separate features collectively in the WinSURF program. Figure 2 shows the 3D reconstruction of the contoured myotome, the skin, and vertebral column. The zigzag pattern of the myotome is evident, as well as indentations where lateral red muscle bands reside beneath the skin along the length of the body.
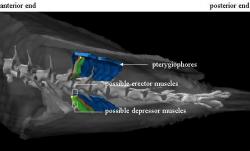
Four muscle groups lie between each pterygiophore; these bones are located between the spines of the dorsal and haemal arches (Figure 3). The arrangement of the erector muscle groups for a single dorsal and anal fin ray in relation to the pterygiophores is depicted in Figure 4, and Figure 5 shows all four groups, two erectors and two depressors, rendered together. The inner bilateral muscle pairs, identified as erector muscle, have a distinct coloration that suggests they may be slow-twitch, oxidative (red) muscle fibers.
Figure 3: Model depicting six sets of pterygiophores, depicted in blue, along the median fins between the neural spines. The ridges on the pterygiophores provide support to the muscles of the dorsal and anal fins. Posterior direction is to the left.
Discussion and Conclusion
The muscle bundles surrounding pterygiophores reconstructed in this study appear to be the erector and depressor muscle groups. These muscles control fin ray movements via ligaments, providing undulatory action in the fins. Distinguishing between the depressor and erector muscles would require in vitro electrical stimulation and finer scale investigation of muscle attachments to the fin ray base, thus it is suggested that this work be pursued to associate muscle groups and their roles in propulsion. However, unpublished preliminary work suggests that the two most interior muscle groups function as the erectors, and the more superficial groups may be the depressors (Christopher Harvey, unpublished observations).
Figure 4: Three-dimensional model showing the relationship between possible sets of erector muscle bundles, depicted in orange and red, along the median fins. These two muscle groups lie adjacent to each other connecting the pterygiophore to a fin ray base. Posterior direction is to the left.
Triggerfish myology differs from that of typical fishes, but seems to also deviate from that described by Winterbottom (1974). Where Winterbottom described two distinct muscle groups between the pterygiophores for each fin ray, thin slice plastination clearly shows four. Therefore, depressor and erector muscle groups observed in this study have a more complex arrangement than previously illustrated. Considering this arrangement in the context of the preliminary findings mentioned above, instead of one erector and one depressor group lying side by side between pterygiophores, it appears that erectors and depressors are arranged along the sagittal plane of the vertebral column, the depressors in the more lateral position, with the erectors lying beneath and medial. These observations reinforce the necessity to examine muscle control and detailed anatomy of these median fin muscle groups. It would be interesting to investigate whether the evolution of four distinct muscle bundles provides increased precision in range, or power of fin ray motion.
The proposed organization of median fin muscles in triggerfish also varies from observations of the dorsal fins of seahorses. The dorsal fin and pectoral fins are the sole means of locomotion in seahorses. Undulation of the dorsal fin allows slower movement, but excellent maneuverability (Consi et al. 2001). Consi et al. (2001) examined dorsal fin myology in these fishes and found an arrangement similar to Winterbottom's (1974) description of balistid musculature in the median fins. Paired inclinator (equivalent to elevators in Winterbottom's description) and depressor muscle groups along the dorsal ridge control the lateral movements of the dorsal fin rays. A third muscle pair, the elevators as referred to by Consi et al. (2001), are present anterior to the inclinators, but only in the first dorsal fin ray.
Figure 5: Dorsal view showing the relationship between possible erector, in orange and red, and depressor muscle groups, depicted in green and yellow. The depressors lie superficially to the erectors, and together they form a group of four bundles associated with each pterygiophore and fin ray.
Although both triggerfishes and seahorses have evolved the use of median fin undulation for propulsion, it is apparent that deviating myology may have resulted. Seahorses use camouflage to blend in with seagrasses and reef, making slow movement with high maneuverability a great asset to avoid predation (Blake 1980). These qualities are also compatible with their reproductive and hunting methods (Lourie et al. 1999; Vincent & Sadler 1995). In contrast, triggerfishes are much more active, spending time in the water column actively searching for prey, defending territories and nests (Kawase 2003; Chen et al. 2001). The need to generate more power while maximizing efficiency, and for precise fin movement at higher speeds may reflect the difference between median fin muscle anatomies of these two groups of fishes.
The control of fins by red muscle groups is vital to sustained swimming abilities in fishes (Mosse & Hudson 1977). Results of this study suggest that red muscle may be recruited to power the median fins in triggerfish. Dissection and plastination of R. rectangulus showed that this species has retained the lateral red muscle, but may also have evolved this muscle type along the median fins, specifically in the suspected erector muscle groups. Red muscle controlling the median fins would allow sustained fin undulation for continuous swimming, and the presence of white muscle in the suspected depressors would allow quick adjustments for swift maneuvers, and bursts of power for increasing speed.
For this study, red muscle groups were designated visually by comparing tissue pigmentation due to myoglobin concentration. The coloration of suspected red muscle groups adjacent to the pterygiophores was comparable to that of the lateral bands; however, histochemical work should be performed to confirm the muscle type. Histochemical staining could also provide information concerning the location of pink muscle, an intermediate between white and red, and the distribution of all muscle types and gradients throughout the trunk. In addition, reconstruction should be continued to examine additional muscle groups and other anatomical features, including connective tissue involved in transmitting muscle force to the fin rays. Increased resolution may be achieved using slices less than 1 mm in thickness, and if appropriate calibration markers are utilized, precise measurements of structures can be acquired.
Three-dimensional reconstruction of muscle groups associated with median fins in R. rectangulus provided an excellent visualization of the spatial relationships between skeletal and muscle structures of this region. Although the use of plastination is becoming increasingly accepted in the fields of medical and veterinary science, its applications to systematics and other biological fields is also significant. Plastination has previously been applied to fishes; Asadi (1998) successfully preserved sturgeon organs, and later Chanet et al. (2005) used plastination to compare kidney anatomy of soleids. Although these studies did not employ ultra-thin slice preparation, its use in comparative anatomy is evident.
Three-dimensional reconstruction can also provide anatomically accurate shape, but can additionally maintain the spatial relationships to other structures and location within the body. Winterbottom (1974) used comparative myology to reveal phylogenetic relationships among the Tetraodontiformes; the use of plastination and 3D reconstruction could offer an opportunity to examine the muscle differences among species with greater detail, aiding in comparative morphology studies to reveal relationships among species.
Ultra-thin slice plastination has shown a remarkable ability to examine the structural features of the triggerfish. Reconstruction of the animal provided a means to study its morphology, while decreasing loss of life by limiting the number of specimens required for dissection. It also allowed manipulation to view the specimen at all angles and select only desired features for rendering. The applications for this preservation method are endless and its use in non-medical fields should be realized and put to use, especially in comparative morphology and systematic studies.
Acknowledgements
The author would like to graciously thank Dr. Scott Lozanoff and Sara Doll at the University of Hawaii for conducting the plastination procedure; Mircea- Constantin Sora at the Medical University of Vienna, Austria for thin sectioning; and Christopher Harvey of Hawaii Pacific University for obtaining and fixing the specimen used for this work. A HPU Technology Enhancement Grant to Dr. Keith Korsmeyer provided computer equipment for this project.
References
Asadi, M.H. (1998) Plastination of sturgeons with the S10 technique in Iran: The first trials.
Journal of International Society for Plastination 13, 15-16.
Baeres, F.M.M. & M. Møller (2001) Plastination of dissected brain specimens and Mulligan-
stained sections of the human brain. European Journal of Morphology 39, 307-311.
Blake, R.W. (1980) Undulatory median fin propulsion of two teleosts with different modes of
life. Canadian Journal of Zoology 58, 2116-2119.
Bond, C. (1996) Biology of fishes. New York, New York: Sauders College Publishing.
Calliet, G., M. Love and A. Ebeling (1986) Fishes: A field and laboratory manual on their
structure, identification and natural history. Prospect Heights, Illinois: Waveland Press, Inc.
Chanet, B., E. Betti, M. Desoutter, C. Guintard & G. Grondin (2005) The anatomy of the
kidney of the Soleidae (Teleostei: Pleuronectiformes): The importance of plastination and interest for the phylogeny of flatfishes. Anatomia, Histologia, Embryologia: Journal of Veterinary Medicine Series C 34, 11.
Chen, T.C., F.F.G. Ormond and H.K. Mok (2001) Feeding and territorial behavior in juveniles
of three co-existing triggerfishes. Journal of Fish Biology 59, 524-532.
Consi, T.R., P.A. Seifert, M.S. Triantafyllou, and E.R. Edelman (2001) The dorsal fin engine of
the seahorse (Hippocampus spp.). Journal of Morphology 248, 80-97.
Douglass, C. & R. Glover (2003) Plastination: Preservation technology enhances biology
teaching. American Biology Teacher 65, 503-510.
Genser-Strobl, B. & M.C. Sora (2005) Potential of P40 plastination for morphometric hip
measurements. Surgical & Radiologic Anatomy 27, 147-151.
Grondin, G. (1998) Plastination: A modern approach to chiropractic teaching. Journal of the
Canadian Chiropractic Association 42, 107.
Henry, R.W. (2005) Using plastinated specimens in teaching veterinary anatomy. Anatomia,
Histologia, Embryologia: Journal of Veterinary Medicine Series C 34, 11.
Kawase, H. (2003) Spawning behavior and biparental egg care of the crosshatch triggerfish,
Xanthichthys mento (Balistidae). Environmental Biology of Fishes 66, 211-219.
Lighthill, J. and R. Blake (1990) Biofluid dynamics of balistiform and gymnotiform
locomotion. Part 1: Biological background and analysis by elongated-body theory. Journal of Fluid Mechanics 212, 183-207.
Lourie, S.A., A.C.J. Vincent, and H.J. Hall (1999) Seahorses, an identification guide to the
world's species and their conservation. Montreal: Project Seahorse, 1-14.
Lozanoff, S. (1992) Accuracy and precision of computerized models of the anterior cranial base
in young mice. Anatomical Record 234, 618-624.
Lozanoff, S. & V.M. Diewert (1989) A computer graphics program for measuring two- and
three- dimensional form change in developing craniofacial cartilages using finite-element methods. Computes and Biomedical Research 22, 63-82.
Lozanoff, S., S. Jureczek, T. Feng & R. Padwal (1994) Anterior cranial base morphology in
mice with midfacial retrusion. Cleft Palate-Craniofacial Journal 31, 417-428.
Maeta, M., K. Uno & R. Saito (2003) The potential of a plastination specimen for temporal
bone surgery. Auris Nasus Larynx 30, 413.
Moody, D. & S. Lozanoff (1998) SURFdriver: A practical computer program for generating
three-dimensional models of anatomical structures using a PowerMac. Clinical Anatomy 11.
Mosse, P. & R. Hudson (1977) The functional roles of different muscle fiber types identified in
the myotomes of marine teleosts: a behavioral, anatomical, and histochemical study. Journal of Fish Biology 11, 417-430.
Moyle, P. & J. Cech (2004) Fishes: An Introduction to Ichthyology. Upper Saddle River, NJ:
Prentice-Hall Inc.
Sora, M.C. & B. Genser-Strobl (2005) The sectional anatomy of the carpal tunnel and its
related neurovascular structures studied using plastination. European Journal of Neurology 12, 380-384.
Sora, M.C., B. Genser-Strobl, J. Radu & S. Lozanoff (2006) Three-dimensional reconstruction
of the ankle by means of ultrathin slice plastination. Clinical Anatomy 19.
Thomas, M., H. Steinke & T. Schulz (2004) A direct comparison of MR images and thin layer
plastination of the shoulder in the apprehension-test position. Surgical & Radiologic Anatomy 26, 110-117.
Van Leeuwen, J. (1999) A mechanical analysis of myomere shape in fish. Journal of
Experimental Biology 202, 3405-3414.
Vincent, A.C.J. and L.M. Sadler (1995) Faithful pair bonds in wild seahorses, Hippocampus
whitei. Animal Behavior 50, 1557-1569.
Von Hagens, G. (1979) Impregnation of soft biologcial specimens with thermosetting resins and
elastomers. Anatomical Records 194, 247-255.
Von Hagens, G. (1986) Heidelberg plastination folder 1985: Collection of all technical leaflets
for plastination. Anatomisches Institut 1. Universität Heidelberg.
Webb, P.W. (1982) Locomotor patterns in the evolution of Actinopterygian fishes. American
Zoologist 22, 329-342.
Webb, P.W. (1984) Form and function in fish swimming. Scientific American 251, 72-82.
Webb, P.W. (1998) Swimming. In: The Physiology of Fishes (ed. D.H. Evans). Boca Raton:
CRC Press, 3-24.
Winterbottom, R. (1974) The familial phylogeny of the Tetraodontiformes (Acanthopterygii:
Pisces) as evidenced by their comparative myology. Smithsonian Contribution to Zoology 155.