Author: Shruti Goel
Institution: George Washington University
Date: February 2007
Abstract
Food-borne bacterial infections pose a significant risk for human populations. The increasing occurrence of antibiotic resistance in bacterial strains has heightened this risk further. This circumstance has led to a renewed interest in traditional treatments for infections. This investigation focused on the bio-protective properties of turmeric, which is a spice that is used in India for extensive medicinal and culinary purposes. The antioxidant and antimicrobial properties of turmeric were investigated using simple, but sensitive chemical and microbiological assays. A hexane extract of turmeric was prepared. The phosphomolydenum and the β-carotene-linoleate methods were used to study antioxidant activities. The phosphomolybdenum assay gave a total antioxidant capacity of 52 mmol α-tocopherol/g. For the β -carotene-linoleate assay, the turmeric extract showed significant antioxidant activity (92.3%). The turmeric extract showed considerable antimicrobial activity as measured by a standard MIC assay. Gram-positive and gram-negative bacteria, some pathogenic, were inhibited at relatively low concentrations of 20micrograms/ml to 100micrograms/ml. Only one pathogenic bacterium, Campylobacter jejuni, proved resistant. The investigation demonstrated that turmeric has potent antioxidant and antimicrobial activities.
Introduction
In a recent study, the CDC concluded that in the United States food-borne diseases account for approximately 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths each year (Mead 1999). Bacterial infections account for about 5 million of these illnesses, which is about 13% of the 38.6 million food-related illnesses caused by known pathogens (Mead 1999). Thus it can be easily seen that food-borne bacterial infections pose a great risk toward the human population. With the rapid increase of antibiotic resistance, there has been an effort in scientific research to find alternate drugs to treat bacterial infections (Desnottes 1997). There has therefore been a renewed interest in the usage of more traditional treatments of infections, including the use of herbal medicine. Therefore, any research elucidating the antibacterial properties of spices and plant extracts could prove to be a promising alternative to those antibiotics, which are becoming ineffectual against bacterial infections.
Curcuma longa Linn., commonly known as turmeric, is a spice used extensively in Eastern cuisine. The root of the plant is normally ground to yield a yellow colored powder, which is then used for various applications. Some of the culinary uses of turmeric are as a coloring and flavoring agent and as a food preservative. Turmeric is also known to have medicinal applications dating back several millennia and has been an important component in the ancient Indian Ayurvedic medicines. Some of its documented modern medicinal uses include its wound healing capacity (Biswas and Mukherjee 2003) and its use for the treatment of inflammation and tumors (Gescher et al. 2005).
Turmeric contains phenolic compounds called curcuminoids that are chemically related to curcumin, the main ingredient of turmeric. These curcuminoids are responsible for the yellow color of the root of the Curcuma longa plant. In fact, it is the curcuminoids that possess all the bio-protective properties of turmeric (Badmaev et al. 2004).
An estimated 2400 metric tons of turmeric are imported into the USA annually, according to the Food and Agricultural Organization of the United States (Gescher et al. 2005). In the USA, turmeric has largely been used for culinary purposes and until recently, its medicinal properties remained unexplored, except, in the few cases documented above. However, recently, several properties of turmeric have begun to be vigorously researched and are opening new venues for its medicinal use. Among these properties are its antioxidant, anti-inflammatory, anti-carcinogenic, antimutagenic, anti-thrombotic, hepatoprotective, and antimicrobial, antiviral and anti-parasitic actions (Badmaev et al. 2004).
Previous research, of interest in the food preservation industry, has focused on the antioxidant properties of the curcuminoids (Badmaev et al. 2004). As Gescher et al. (2005) point out in a recent review on the medicinal applications of curcuminoids, much of this research involves animal studies, which are lengthy and costly. The present investigation sought to establish that crude turmeric extracts have potent antioxidant and antimicrobial capacities and that turmeric could be a potential alternative to common antibiotics. This study used simple but sensitive in vitro chemical and microbiological assays to assess the antioxidant and antimicrobial activities of crude turmeric extracts.
Materials and Methods
Materials
The turmeric powder was obtained from a local Patel Brothers Indian grocery store. The bacterial strains used in the investigation are presented in Table 1.
Table 1 Bacterial Strains used in this investigation3
Extraction of turmeric and curcumin enrichment
Twenty grams of turmeric powder were slowly stirred into 100 ml hexane for 15 minutes. The mixture was filtered and the extraction was repeated two more times in 100 ml of hexane each time. Solvent was removed from the combined extracts through evaporation (Jayaprakasha et al. 2002). The resulting turmeric extract was in the form of viscous oil, rich in curcumin. To reduce the viscosity, 0.5 ml of DMSO was added to the turmeric extract to give a solution concentration of 2mg/ml in DMSO.
Antioxidant Activity
To evaluate the total antioxidant capacity of the turmeric extract, the phosphomolybdenum method by Prieto et al. (1999) was followed. This method is based upon the reduction of Mo (VI) to Mo (V) by the sample and the subsequent formation of green phosphate/Mo (V) complex, which has an absorption optimum of 695 nm (Prieto et al. 1999). For the assay, 0.1ml of the sample (100 mg) was added to an Eppendorf tube containing 1 ml of the reagent (0.6 M sulfuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). Similarly, a 0.1 ml aliquot of DMSO was also combined with the reagent to act as the control for the determination. After the solutions were added to the tubes, they were capped and incubated at 95°C for 90 minutes. After the solutions cooled to room temperature, their optical density (OD) was measured at 695 nm. The samples were diluted in DMSO. The total antioxidant capacity was determined in terms of mmol of α-tocopherol/g of turmeric extract, as recommended by Prieto et al. (1999).
A second independent method used to evaluate the antioxidant properties of turmeric was the β-carotene-linoleate model as described by Jayaprakasha et al. (2002). In a Corex glass tube, 0.5 ml chloroform, 5 ml β-carotene (0.2 mg), 20 ml linoleic acid (20 mg) and 0.2 ml Tween-40 (polyoxyethylene sorbitan monopalmitate) (200 mg) were combined. The tube was then incubated in a 50°C evaporator for 10 minutes to remove the chloroform. The resulting mixture was diluted with double distilled water and 4 ml aliquots of this solution were then combined with the samples as follows. The control consisted of 0.2 ml of ethanol and 0.2ml of the sample was prepared with 0.15 ml ethanol and 0.05 ml of the turmeric extract. At 470 nm, the (OD) of the control as well as the extracts and a BHA standard were recorded at time t = 0 minutes and subsequently at half-hour intervals until time t = 90 minutes. Between determinations, the samples were incubated in a 50°C water bath. Three independent determinations were made. The antioxidant activity (AA) was measured based on the ability of the samples to prevent the bleaching of the β-carotene. The AA was quantified using the formula AA = 100 [1-(Ao- At)/(Aoo Aot)] where Ao is the OD of the sample at time t = 0 minutes and At is the OD of the sample at time t = 90 minutes. Similarly, Aoo and Aot represent the OD of the control at time t = 0 minutes and t = 90 minutes, respectively.
Antimicrobial Activity
To test the antimicrobial properties of the turmeric extract, the minimum inhibitory concentration (MIC) method by Abdulrahaman (1986) was followed. A colorimetric assay using purple broth was carried out in 3 ml tubes. Initially, all bacteria (Table 1) were grown in purple broth and it was found that for all of the strains, if the solution turned yellow, it indicated growth but if it remained purple, no growth had occurred. The turmeric extract was diluted to 100 micrograms/ml, 40 micrograms/ml, 20 micrograms/ml and 10 micrograms/ml concentrations in DMSO to estimate the MIC. 50 ml of bacterial cells (grown in LB broth to a density of 2 X 108 c.f.u./ml) were combined with 50 ml of each of the dilutions of the extract. A control consisting of cells combined with DMSO was included in all evaluations. The tubes were incubated for 30 minutes at 37°C. Then, 1 ml of purple broth plus 1% dextrose were added to the tubes and they were shaken at 37°C for 24 hours. The experiment was run in triplicate.
In order to determine the minimum killing concentration (MKC), the bacterial cells from the colorimetric assay above were streaked on to Eosin Methylene Blue (EMB) containing plates and incubated at 37°C for at least 24 hours. After incubation, based on the presence or absence of bacterial growth, the strains were recorded as bacteriostatic or bacteriocidal at the concentrations of turmeric extract indicated above.
Data presentation and Statistics
Data for the antioxidant assays are the means +/- Standard Deviation (SD). Statistical analysis was carried out using the Student's t-test and p-values were based on multiple (3-4) repeated tests.
Results
Figure 11. Total antioxidant capacity of turmeric extract compared to green tea, garlic and two other spice extracts. The assay compared extracts prepared using an identical protocol. The quantitative antioxidant capacity of turmeric (H1), green tea, garlic and other extracts was measured spectrophotometrically based on the reduction of Mo (VI) to Mo (V) and the subsequent formation of green phosphate/Mo (V) complex measured at 695 nm. Data are means +/- SD. Control reactions (containing solvent only) showed minimal conversion of Mo (VI) to Mo (V) under standard reaction conditions (equivalent to less than 1mmol α-tocopherol/g).
Antioxidant Activity
We used a simple hexane extraction of turmeric to yield a preparation rich (> 70% v/v) in curcumin for our tests. The total antioxidant capacity of turmeric was evaluated by the phosphomolybdenum method devised by Prieto et al. (1999). Figure 1 shows the total antioxidant activity expressed in mmol α-tocopherol/g of the turmeric extract compared with extracts of garlic, green tea and two other spices made in the same way for comparative purposes. Based on this assay, the turmeric extract had a total antioxidant capacity of 52 (+/- 2.0) mmol α-tocopherol/g. As expected, the values for garlic and green tea were very high (370.8 (+/- 4) mmol α-tocopherol/g and 166(+/- 0.6) mmol α-tocopherol/g, respectively). Garlic and green tea are known to have high antioxidant activities. In green tea (Chen et al. 1998), catechins have been identified as the major source of antioxidant properties whereas in garlic (Madsen and Bertelsen 1995), flavonoids, and the sulfur-containing compounds allicin, alliin, ajoene have all shown to be highly potent antioxidants. The turmeric extract compared favorably (about 31% of the green tea value). The extraction of antioxidants from spices depends on the polarity of the solvent. Based on numerous studies (summarized in Gescher et al. 2005) extracts of spices (including turmeric) seem to yield more effective antioxidant activities in bulk oil systems. Turmeric contains both water-soluble compounds (such as turmerin), essential oils (turmerones, atlantones and zingiberene) and a group of phenolic compounds known as curcuminoids, of which curcumin is a major constituent (Gescher et al. 2005).
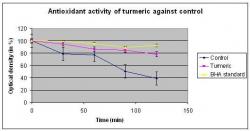
Figure 2. Antioxidant activity using the β-carotene-linoleate model2. The graph shows the decrease in absorbance of β-carotene in the presence of turmeric extract, the BHA standard and in the absence of either compound (control) with the coupled oxidation of β-carotene and linoleic acid. The Student t-test indicates p
The results from the β-carotene-linoleate model are presented in Figure 2. It shows the bleaching (decrease in OD) of the β-carotene compared with butylated hydroxyanisole (BHA standard). Figure 2 shows the decrease in absorbance (due to bleaching) of β-carotene in the presence of BHA or curcumin-enriched turmeric extract. The presence of BHA and turmeric extract prevented the bleaching compared to a control of β-carotene to different degrees. After 90 minutes, β-carotene bleaching was reduced from 50 (+/- 3.2)% in the absence of turmeric extract (control) to 15.7 (+/- 5.7)%, a 3-fold reduction in oxidation of β-carotene when turmeric extract was incorporated into the assay (Figure 2). The results are comparable to BHA, routinely used as a standard antioxidant, which also reduced β-carotene bleaching 3-fold over 90 minutes (18.8% +/- 4.5). When the formula defined by Jayaprakasha et al. (1999) was applied to the data, the turmeric extract showed 92.3% antioxidant activity compared to the BHA standard with only 78.7% activity at a concentration of 100 micrograms/ml (Table 2). Also, for comparison, the antioxidant activity for garlic and green tea measured by the same assay, were computed and found to be much less compared to the turmeric extract.
Table 2. Comparative percent Antioxidant activity using the β-carotene-linoleate model4. Antioxidant activity was quantified using the formula described by Jayaprakasha et al. (2002).
The apparent discrepancy between the results from the phosphomolybdenum method and the β-carotene-linoleate model can be due to the fact that the phosphomolybdenum method determines the total antioxidant capacity whereas the β-carotene-linoleate model only measures antioxidant activity as it relates to the bleaching of the β-carotene. In fact, it has been suggested that the antioxidant activity in vivo, is best measured by the β-carotene-linoleate model (Jayaprakasha et al. 1999).
The active antioxidant compounds in turmeric have been conjectured to be a group of phenolic compounds including curcumin (Bernd et al. 2002). Curcumin has been shown to scavenge superoxide anion radicals and hydroxyl anions (Absan et al. 1999). However, the same author points out that curcumin, in common with many other dietary phytochemicals, may have pro-oxidant effects as well, which are dependent on dose and chemical environment.
Table 3. Antimicrobial Testing: MIC5 values represent the minimum amount of extract (measured in micrograms/ml) required to inhibit the growth of bacteria under standard incubation conditions of 37ºC and 24 hours of incubation period.
Antimicrobial Activity
The MIC values are as shown in Table 3. E. coli and E. aerogenes were most sensitive to the turmeric extracts since their growth was inhibited at 20 micrograms/ml while the K. pneumoniae and S. enteritidis were inhibited at higher concentrations (100 micrograms/ml) of the extract. The multiple drug-resistant S. aureus strain required 40 micrograms/ml of the turmeric extract for inhibition, the MKC measurements, unfortunately, gave inconsistent results. Campylobacter jejuni growth showed no evidence of inhibition up to 100 micrograms/ml.
The susceptibility of many of the organisms to the turmeric extracts, including pathogenic strains of E. coli, E. aerogenes, K. pneumoniae and S. enteritidis, is very interesting, considering the overwhelming incidence of antibiotic resistance. Most significantly, the S. aureus strain used in this study (isolated from GW Hospital) was a multiple drug resistant (MDR) strain resistant to methicillin and vancomycin. In addition, some of the bacteria, such as S. enteritidis and E. coli, are widely implicated in cases of food poisoning in the US and worldwide (Mead 1999). Turmeric has traditionally been used as a food preservative. Traditionally too, plant materials commonly used to treat infections or as food preservatives, do not aim at using specified extracts. However, we have shown that the curcumin-enriched extracts here are very effective at inhibiting the growth of most of the bacterial strains at very low concentrations. For comparison, a study of Chinese herbs reported that extracts of Huangqin (Scutellaria sp.), a Chinese medicinal herb, inhibited K. pneumoniae at 200 micrograms/ml (Franzblau and Cross 1986). The turmeric extract was able to inhibit the growth of the same strain of the bacterium at a significantly lower concentration of 100 micrograms/ml.
Discussion and Conclusion
Turmeric has been found to be a rich source of beneficial phenolic compounds known as curcuminoids. The properties of the curcuminoids as potent antioxidants have also been widely documented (Gescher et al. 2005; Badmaev et al. 2004; Miquel J. et al. 2002; Absan, H. et al. 1999). They have been found to scavenge free radicals, particularly those responsible for the cardiovascular disease lipid peroxides (Badmaev et al. 2004). Curcuminoids have also been shown to prevent the formation of free radicals (Badmaev et al. 2004).
In our study, we used crude extracts of turmeric root, rather than partially or fully purified curcuminoid fractions, to mimic the release of active components expected in cooking. We showed that such extracts contained significant antioxidant activities comparable to purified curcuminoid preparations. Our crude extracts gave an antioxidant capacity, as measured by the phosphomolybdenum assay of 52 mmol α-tocopherol/g, about 10-fold less than that reported by Jayaprakasha et al. (2002) who used curcumin-removed turmeric oil. However, when assayed by the β-carotene-linoleate model (which better approximates in vivo activity), our extracts yielded results comparable to previous in vitro studies using purified curcuminoids (Jayaprakasha et al. 2002) and were as effective as butylated hydroxyanisole (BHA) at the same concentrations. Furthermore, our results support other findings that combinations of curcuminoids, rather than each individual component, are far more effective as antioxidants (Desnottes 1997; Franzblau and Cross 1986).
Badmaev at al. (2004) have pointed out that spices have important antibacterial benefits. The phytochemicals they contain are involved in the plant's defense system against insects, fungi and other parasites. It is, therefore, not surprising that these chemicals also have important antimicrobial effects, an important consideration given the increasingly large numbers of food-borne infections and intoxications reported in the United States and elsewhere. The antimicrobial effects of turmeric have been reported by others (Badmaev at al. 2004) but here we show that crude extracts were able to inhibit the growth of most of the Gram-positive and Gram-negative bacteria used in this study at concentrations expected to be present in traditionally prepared food. In addition, our finding that turmeric extracts are effective antimicrobial agents against some multiple drug-resistant bacteria may have important implications for preventing or treating infections caused by these microorganisms.
Acknowledgements
I would like to thank Dr. Morris, George Washington University, Washington DC for his guidance and encouragement in this investigation.
References
1. Mead, Paul S. et al. (1999) Food related illnesses and death in the United States. Emerging Infectious Diseases 5, 607-725.
2. Desnottes, Jean-Francois (1997) New targets and strategies for the development of antibacterial agents. Microbiology 35, 134-140.
3. Gescher, A. et al. (2005) Curcumin: The story so far. European Journal of Cancer 41(13), 1955.
4. Biswas, Tuhin Kanti and Mukherjee, Biswapati (2003) Plant medicines of Indian origin for wound healing activity: a review. Lower Extremity Wounds 2 (1), 36-37.
5. Badmaev, Vladimir et al. (2004) Curcuminoids: bioprotectant compounds from turmeric. Sabinsa Corporation, 2.
6. Jayaprakasha, G. K. et al. (2002) Evaluation of antioxidant activities and antimutagenicity of turmeric oil: A byproduct of curcumin production. Z. Naturforsch, 829-30.
7. Prieto, P. et al. (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem 269, 337-341.
8. Abdulrahaman, E. M. Phytochemical and biological studies of the bark Sclerocarya biarrae (A. Hochst). Department of Pharmacognosy and Drug Development, MSc Thesis, Ahmadu Bello University, Zaria, 1986.
9. Maron, D. M. and Ames, B. N. (1983) Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113, 173-215.
10. Ikken, Y. et al. (1999) Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames test. J. Agric. Food Chem. 47, 3257-3264.
11. Chen, Z.Y. et al. (1998) Antioxidative activity of green tea catechin extract compared with that of rosemary extract. JAQCS. 75, 1141-1145.
12. Madsen, H. L. and Bertelsen, G. (1995) Spices as antioxidants, a review. Trends Food Sci. Techn. 6, 271-277.
13. Miquel J. et al. (2002) The curcuma antioxidants: pharmacological effects and prospects future clinical use. A review. Arch. Gerontol. Geriatr. 34, 37-46.
14. Absan, H. et al. (1999) Pro-oxidant, anti-oxidant and cleavage activities in DNA of curcumin and its derivatives dimethoxycurcumin and bisdimethoxycurcumin. Chem. Biol. Interact. 121, 161-175.
15. Franzblau, S. G. and Cross, C. (1986) Comparative in vitro antimicrobial activity of Chinese medicinal herbs. J. Ethanopharmocol. 15 (3), 279-88.