Ryeim Ansaf
When it first developed, 3D printing technology took the world by storm. Students and arts and crafts fans were printing all kinds of creations—from miniature Eiffel towers, to toys, to friendship gifts, and even medical devices. But where did science, engineering, and medicine truly merge with this technological advancement?
3D bioprinting of organs has become a reality, and there are thousands of publications surrounding the field. The need for 3D bioprinting can be associated with many factors, including the increase in human lifespan, with a 6-years increase in lifespan between 2000 and 2019 alone. At the root of increasing lifespan is the biological limitation of the human body. An increase in the chronological age of humans is placing pressure on the biological age and health of individuals, driving the need for regenerative strategies in medicine that focus on rebuilding lost health. Regenerative medicine is defined as the interdisciplinary field that encompasses engineering and life sciences in promoting regeneration, restoration of lost function, and injury repair. 3D bioprinting, along with stem cells, has emerged as a leading method of regeneration over the past decade.
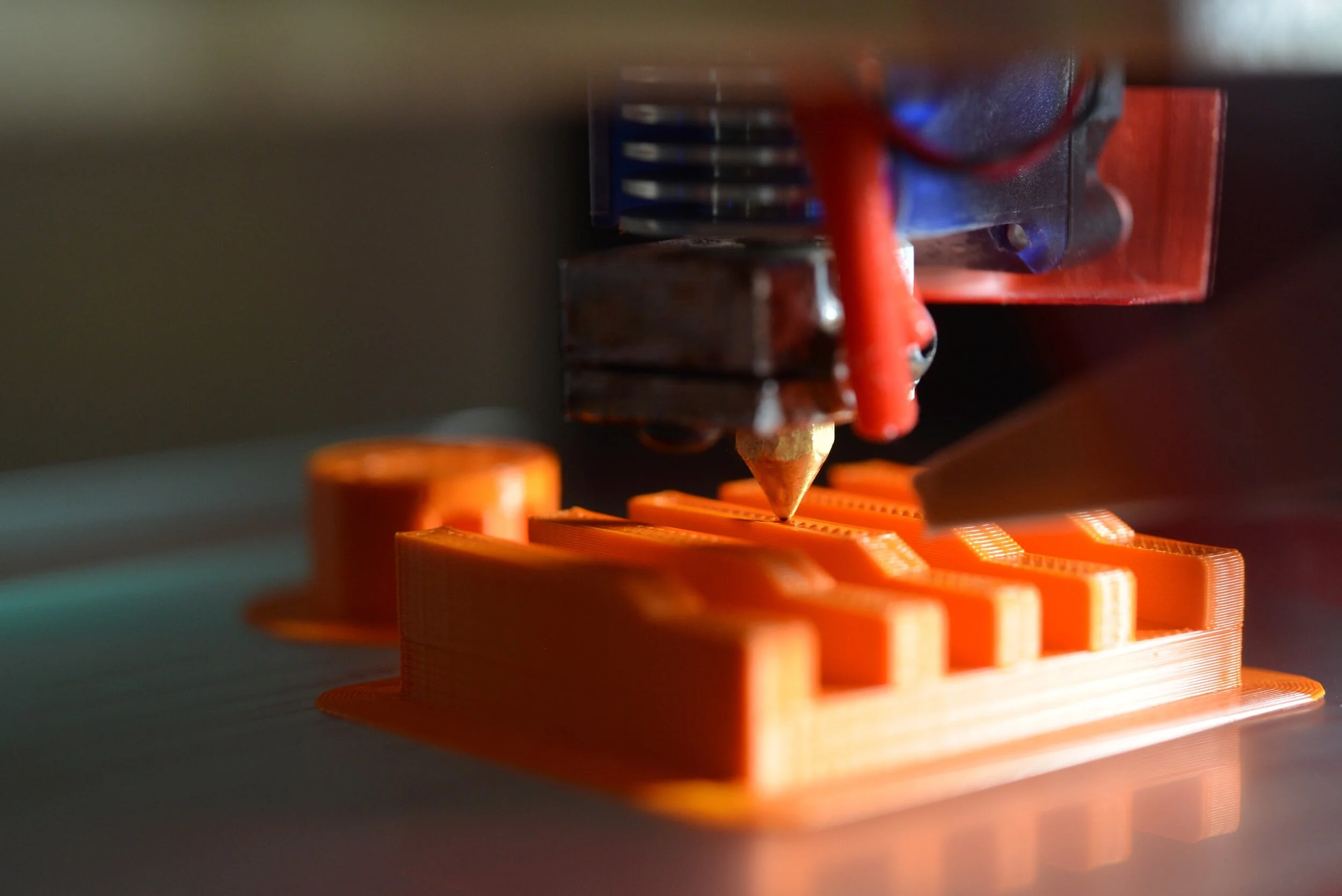
A research group in the Sungkyunkwan University School of Medicine in Suwon, Republic of Korea have printed muscle tissue through a photo-crosslinking method in their manuscript “3D Bioprinting Using a New Photo-crosslinking Method for Muscle Tissue Restoration”, published in the journal npj Regenerative Medicine. The inspiration for the new photo-crosslinking strategy was due to limitations in current cell-containing hydrogel models for tissue engineering like poor cell adhesion. Through the usage of an optic-fiber assisted bioprinting (OAB) process, the researchers were able to crosslink methacrylate hydrogels, which allowed for biofunctionality. For this process, the selection of printing conditions is important as they affect the mechanical properties of the bioink. For example, the researchers emphasized that the stiffness of most bioinks is enhanced post-UV exposure. Other properties analyzed by the researchers include storage modulus, structural stability, and tensile stress-strain curves. The researchers successfully created a methacrylate gelatin (Gelma), collagen, and decellularized extracellular matrix that they used to induce skeletal muscle regeneration. Gelma constructs were made using C2C12 (an immortalized mouse myoblasts cell line) and human adipose stem cells for regeneration. Due to increased biofunctionality, the researchers observed better cell alignment and myogenic activity compared to ones printed using conventional non-OAB methods.
The researchers also explored the effects of certain method-induced parameters such as temperature effects on gelation, and UV-exposure conditions on the compressive modulus of the Gelma bioink. These characteristics are important as they can affect the structural stability of the model, both in vitro and in vivo. Additionally, based on the mechanical properties, the printing method could change. For example, using a stiffer bioink will require more pressure on an extrusion printer and impact the shape of the printed construct. Additionally, the researchers observed that increasing the distance between the optic fiber to nozzle distance improves the circularity of the printed construct and allows for more uniform prints.
Some challenges that remain unresolved are the true functionalization of 3D bioprinted tissues and the creation of a bioink that encompasses all the qualities of native tissue, including mechanical strength, cell adhesion, differentiation, water regulation, and many others. Additionally, since the field of 3D bioprinting is completely new, we are challenged by limitations in funding, pre-existing models, and even regulations surrounding implantation of the 3D bioprinted constructs in vivo and in vitro.
The significance behind this project is very prominent in the field of medicine, especially with its implementation in vivo using a regenerative mouse model used to study muscular defects. The in vivo 3D bioprinted model created by the researchers significantly induced muscle regeneration without any topographical cues. This means the model can be used for fabrication of cell-laden constructs for various tissue engineering constructs, such as blood vessels and nerve grafts. Overall, the successful implementation of the model in vivo serves as proof-of-concept that 3D fabrication is possible through the OAB method proposed by the researchers.
References:
WHO methods and data sources for life tables 1990-2019 (Global Health Estimates Technical Paper WHO/DDI/DNA/GHE/2020.1)
Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):14452-9. doi: 10.1073/pnas.1508520112. PMID: 26598661; PMCID: PMC4664309.
Lee, J., Lee, H., Jin, EJ. et al. 3D bioprinting using a new photo-crosslinking method for muscle tissue restoration. npj Regen Med 8, 18 (2023). https://doi.org/10.1038/s41536-023-00292-5