terrah garner
You walk into a cutting-edge laboratory built to study the complexity of the human population, excited to participate in a new research study. But as you glance around, you think "whoa." Out of everyone in the lab, there is only one race and ethnic group represented. It doesn't feel like it portrays society as a whole and there isn't much diversity. You start to wonder if their study can accurately represent human experience without incorporating a variety of people.
When you consider health conditions that can affect a large number of people, it becomes clear why diversity is so important in study. Take high blood pressure, commonly known as hypertension. It's a major deal and, for some people, even potentially fatal. Mendelian randomization, a technique that uses gene variation to assess cause and effect relationships, has revealed a relationship between high blood pressure and cardiovascular illness. Researchers investigated the effects of genetic variations on blood pressure levels, which can determine the likelihood of developing heart issues. In other words, if you have particular variations in the genes related to blood pressure, you may be more prone to cardiac problems.
To better understand the genetic factors underlying high blood pressure in people from a multitude of backgrounds, scientists conduct genome-wide association studies (GWAS) involving a plethora of racial and ethnic groups. GWAS is an interesting methodology that examines genetic markers throughout a person's genome to discover links between certain genetic variations and variables such as blood pressure levels. In a process similar to a genetic treasure hunt, researchers search the entire genome for hints (genetic markers) that can shed light on why certain people are prone to high blood pressure. In return, this allows researchers to determine how genetic variables affect high blood pressure in various groups by doing GWAS with a mixture of people from various backgrounds.
Using the findings from GWAS, scientists have constructed what are known as Polygenic Risk Scores (PRSs) for hypertension and other blood pressure measurements. These PRSs take into account multiple genetic variants and can help predict blood pressure-related outcomes, including the rate of blood pressure increase. Additionally, these PRSs have demonstrated their ability to predict the occurrence of coronary heart disease and stroke.
Yet, after this scientific breakthrough many questions still stand. In their study, Kurniansyah et al. attempted to address uncertainty by answering pivotal questions in their research publication to nature.com titled "Evaluating the use of blood pressure polygenic risk scores across race/ethnic background groups.” The main question was: should we use a Blood Pressure Polygenic Risk Score (BP PRS) in clinical settings to identify individuals at high risk of hypertension or cardiovascular disease? If the answer is yes, how can we use this information to guide early intervention through drug treatment or lifestyle changes for those at the highest genetic risk?
While addressing these questions, it’s important to consider the potential implications of using Polygenic Risk Scores (PRSs) developed as a result of data primarily from European or White populations as a clinical tool. There is a concern that this approach may contribute to healthcare inequities. This is because the benefits of using PRSs to identify individuals at risk of future diseases might primarily favor White individuals, potentially exacerbating existing disparities in healthcare.
When examining the United States, we can see that there are significant varieties in global ancestries, including the Hispanic/Latino population and the Black and African American population. However, genetic studies on blood pressure have been disproportionately focused on specific populations, which in turn can lead to a lack of minority representation in genome wide association studies.
To address this issue, it’s crucial to assess the performance of PRSs in multi-ethnic populations, including mixed populations like those represented in the Trans-Omics in Precision Medicine Initiative (TOPMed), an NIH-founded initiative that was founded with the goal to transform healthcare by taking into account individual variability in genes, environment, and lifestyle. However, genetic research efforts aimed at investigating the genetic basis of blood pressure have not been equally distributed across different populations, resulting in a scarcity of GWAS focusing on blood pressure within Hispanic/Latino and Black populations. Previous research has explored the generalizability of PRSs for blood pressure traits from White populations to Hispanic/Latino and Black Americans. In their study, Kurniansyah et al. found that individuals of Hispanic/Latino and Black descent face a higher likelihood of experiencing elevated blood pressure (BP). The study's results indicate that when using genetic information primarily derived from White individuals in GWAS, the accuracy of predicting blood pressure in Hispanic/Latino individuals was concerningly low. Even when attempting to improve the predictions by utilizing GWAS results specifically from Hispanic/Latino participants to choose genetic variants or calculate weights, the performance remained less than optimal.
In simpler terms, this means that the genetic information collected from one ethnic group (White individuals) was not effective in accurately predicting blood pressure levels in another ethnic group (Hispanic/Latino individuals). Even when trying to adapt the data to better fit the Hispanic/Latino population, the predictive power of the model did not improve. These findings highlight the importance of considering the genetic diversity among different ethnic groups to develop more precise and effective predictors for health-related traits like blood pressure.
However, using GWAS meta-analysis as the basis for PRSs has been criticized for potentially overlooking population-specific genetic effects. It also raises questions about selecting a single reference-panel population to compute linkage disequilibrium, which is the correlation between genetic markers (SNPs) used to select or adjust the effects of independent blocks of SNPs.
In their study, Kurniansyah and their team investigated how our genes may impact blood pressure in people from various racial and ethnic backgrounds. They didn't just focus on one group, like white individuals, but included diverse populations.
To explore this, the researchers used existing data from different sources, including information from various biobanks and genetic studies. Instead of creating new genetic scores, they analyzed existing data to see how genes might influence blood pressure in different groups.
In examining PRS performance in these backgrounds, the primary goal was to understand how using standard PRS methods for blood pressure may affect health equity. The approach complements efforts to improve PRS methodology and support research in diverse populations.
The study examined people from different biobanks (TOPMed, UK Biobank, MGB Biobank, and All of Us) and found notable differences in their blood pressure levels. Interestingly, the variance in blood pressure was lowest among White participants, higher among Hispanic/Latino and Asian individuals, and highest among Black participants. The variance in blood pressure for Black participants was up to 25% greater than for White participants.
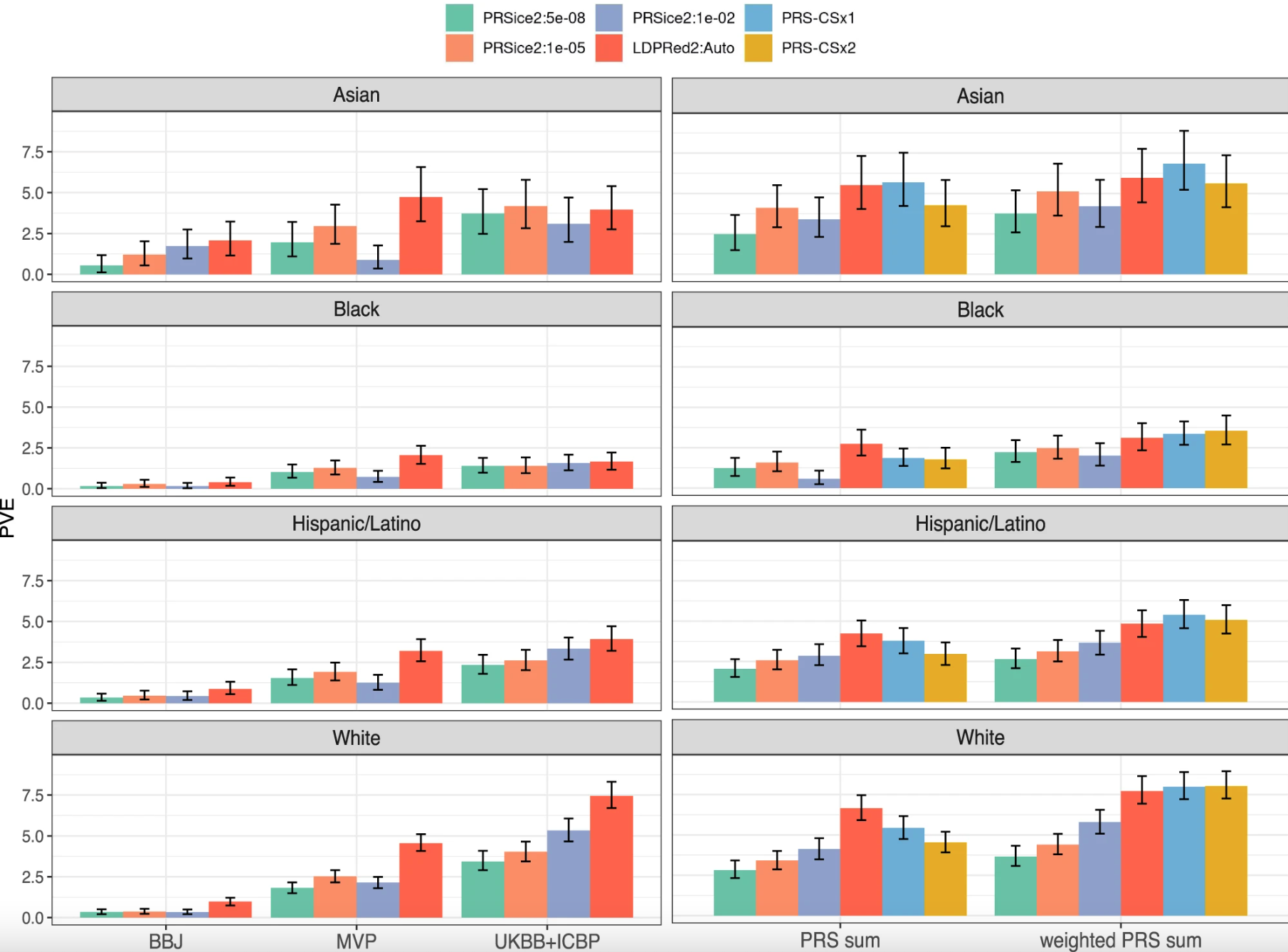
Fig. 2: SBP variance explained by compared SBP PRSs. Image sourced from “Evaluating the use of blood pressure polygenic risk scores across race/ethnic background groups,” found at https://www.nature.com/articles/s41467-023-38990-9
Figure 2 shows how genes affect blood pressure in different groups of people based on their race and ethnicity. Each bar represents how much genes can influence blood pressure in that specific group.
The graph has two columns. In the left column, the bars show how genes from one study affect blood pressure. In the right column, the bars show how genes from multiple studies are combined to affect blood pressure.
The colors blue and yellow represent two different ways of combining genes from these studies. Blue is one method, and yellow is another. These methods use data from different studies instead of using one particular study.
This graph helps us understand how genes can predict blood pressure in different racial and ethnic groups, and how combining data from multiple studies can improve this prediction.
Fig. 3: DBP variance explained by compared DBP PRSs. Image sourced from “Evaluating the use of blood pressure polygenic risk scores across race/ethnic background groups,” found at https://www.nature.com/articles/s41467-023-38990-9
Figure 3 shows a graph about how genes affect diastolic blood pressure in different groups of people based on their race and ethnicity. Each bar on the graph represents the amount of influence that genes have on diastolic blood pressure in that specific group.
The graph has two columns. In the left column, the bars in green and orange show how genes from a single genetic study affect diastolic blood pressure. In the right column, the bars in red and purple show how genes from multiple genetic studies are combined to affect diastolic blood pressure.
The colors blue and yellow represent two different ways of combining genes from these multiple studies. Blue is PRS-CSx1, and yellow is PRS-CSx2. PRS-CSx1 uses data from a study called MVP, while PRS-CSx2 uses data from COGENT, instead of using data from an African ancestry study.
This graph helps to understand how genes can predict diastolic blood pressure in different racial and ethnic groups.
Additionally, The study looked at where the TOPMed-BP participants' ancestors came from. It found that Black participants had ancestors from Africa and Europe, while Hispanic/Latino participants had ancestors from Europe, Amerindian, and Africa. They also found some similarities to European ancestry in Middle Eastern participants.
So, what does that mean? It means that these findings underscore the genetic diversity and complexity of blood pressure traits among different populations, emphasizing the importance of considering ancestral backgrounds in blood pressure-related studies.
To conclude, hypertension poses a significant healthcare risk, and genetic research has identified links between blood pressure and cardiovascular disease. Polygenic Risk Scores (PRSs) have shown improved accuracy in predicting blood pressure-related outcomes. However, these studies also highlight the healthcare disparities present among minority communities. It’s essential for agencies considering the use of PRSs in clinical settings to address these disparities. Current research primarily focuses on specific populations, exacerbating existing inequalities and making it difficult to tackle them effectively. It’s crucial to evaluate the performance of PRSs in diverse populations to ensure accurate risk prediction. The results of this study should serve as an action call for future research efforts to include diverse populations to further promote equity in hypertension accession and treatment.
References:
Kurniansyah, Nuzulul, et al. “Evaluating the Use of Blood Pressure Polygenic Risk Scores across Race/Ethnic Background Groups.” Nature Communications, vol. 14, no. 1, 2 June 2023, p. 3202, www.nature.com/articles/s41467-023-38990-9, https://doi.org/10.1038/s41467-023-38990-9. Accessed 19 July 2023.