SEPTIA NURMALA
IMAGE SOURCE: https://pubs.acs.org/doi/abs/10.1021/acscentsci.1c01080#
When Cy-bug—a robotic, buglike virus—got stranded in the Sugar Rush world, the protagonists faced some serious difficulty finding it. The thick sugar particles in the atmosphere jammed their sensors making it impossible to spot this evil creature. While that can happen in a Wreck-It Ralph universe, the opposites are true in the real world. A recent paper published in ACS Central Science showed a successful development of SARS-CoV-2 detection kit with the help of sugar mixtures.
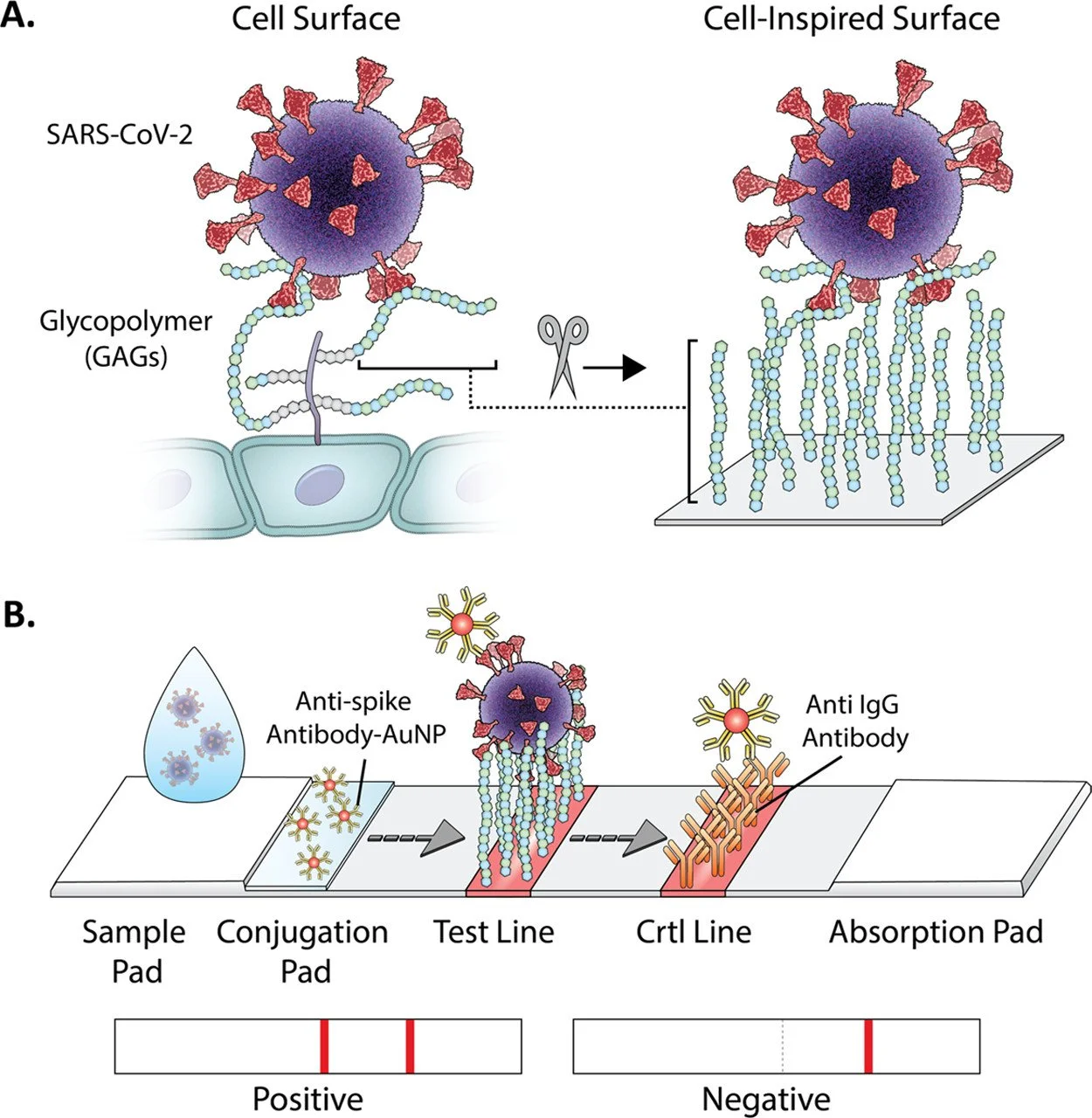
A collaborative effort by University of North Carolina Chapel Hill and University of California San Diego led to the invention of GlycoGrip, a new type of lateral flow test that incorporates glycocalyx. Present on the surface of epithelial cells as a macromolecule, glycocalyx consists mainly of glycoproteins, glycolipids, and proteoglycans. The main role of these polymers is to make contact with other cells and pass down the signal inside. Unfortunately, this sugary net can act as a double-edged sword when there is an infection: glycocalyx facilitates pathogen entry into host cells when exposed. SARS viruses, including SARS-CoV-2 for example, take advantage of this feature to infect the lungs.
Evidence suggests SARS-CoV-2 hijacks the angiotensin converting enzyme 2 (ACE 2) to enter the lungs, but the help of glycocalyx in this whole process is worth mentioning. The viral spike—a glycoprotein that sticks out on the surface of the viral cell, serves as an antennae for the virus and utilizes glycans to stabilize the binding between the spike and ACE 2. In fact, evolutionary biology points to glycosaminoglycan (GAG) binding sites on the spike as a tool necessary to sustain the virus’ life.
GAGs are a type of proteoglycans that consist of heparan sulfate (HS) and chondroitin sulfate (CS). Both HS and CS are made up of repeating uronic acid and N-acetylglucosamine with different degrees of sulfation, a feature that makes the two of them varied and complex. This variation, however, makes GAGs unfavorable to design because many analytes bind to one site and, thus, render it unspecific.
In the early phase of designing the GlycoGrip, the authors needed to confirm which elusive GAG structures bind to the virus spike and how it happens. “By using atomic-level views of the spike protein, we were able to identify key binding sites for the glycocalyx sugar polymers and unlock how these sugars adapt to different spike conformations,” explained Dr. Rommie E. Amaro in UNC's press release, a co-corresponding author on the study. Dr. Amaro, who was in charge of the computational simulations aspect, modeled the structure of GAG as well as constructed a new HS model in silico.
After determining GAG sequences, she and her team continued on to sort out the importance of GAG’s interaction with its predicted binding sites since different conformations of spike can hinder or facilitate the binding itself. Two new sites were found and validated in which GAG interacts with both the receptor binding domain and the cleavage site; two different domains in the spike that are connected by a ridge. This interesting result once again reaffirms that multivalent binding mode is one of GAG’s unique abilities that underlies the fundamental theorem of this research.
Not only is this recursive ensemble-based docking method useful for uncovering the binding sites, it also allows the researchers to elucidate the mechanism behind the interactions between GAG and spike. They found that a compensatory mechanism exists to accommodate the multivalent binding mode of GAG. One particular site, posited to be a hotspot for GAG, changes conformations to facilitate multiple interconverting binding modes. Moreover, glycans present on the spike’s surface have contradictory actions. They shield one of the binding sites for GAG, yet when other binding sites change their shape, these glycans are affected and move away to help secure GAG-spike interactions on that site.
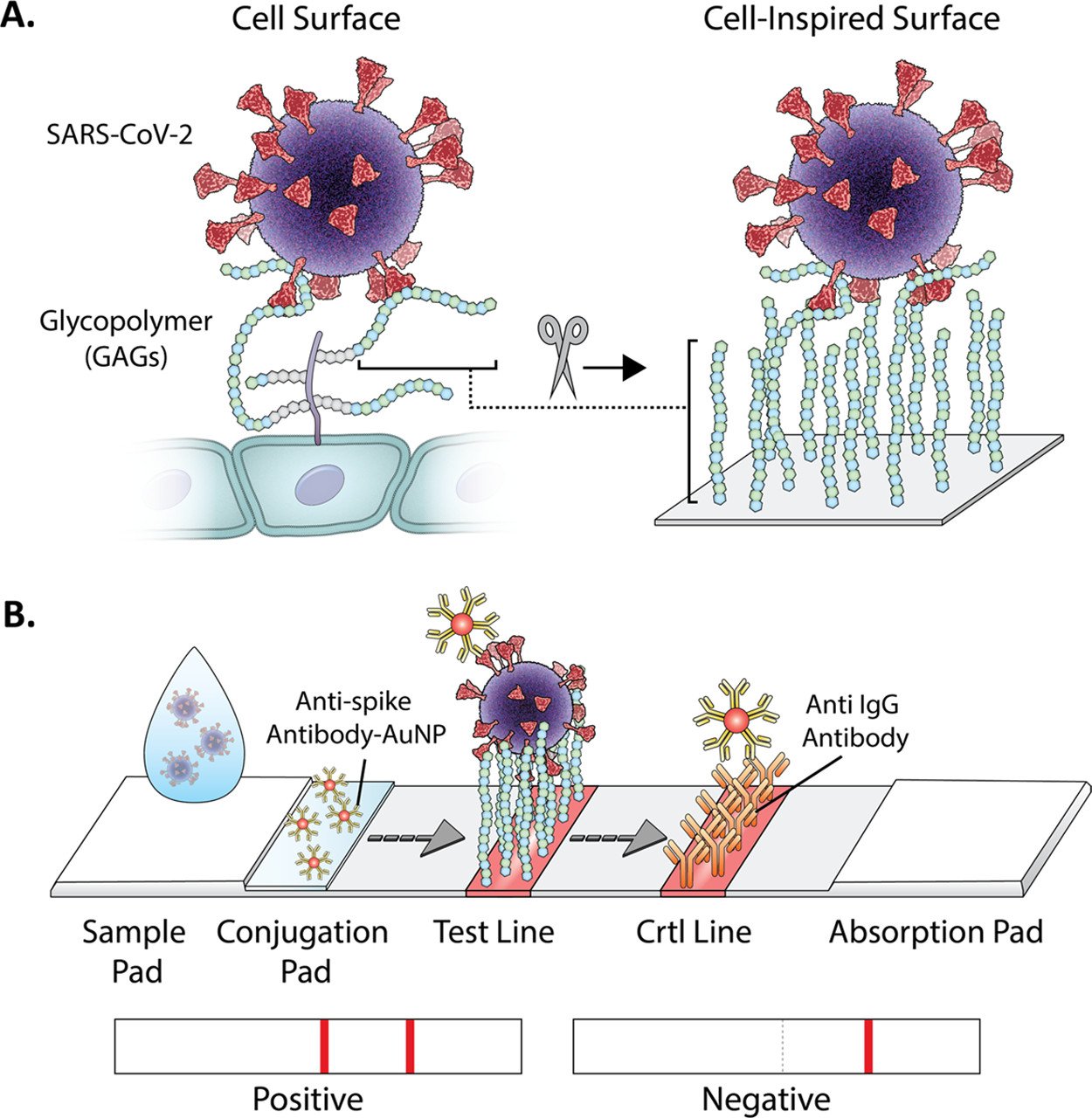
SARS-CoV-2 lateral flow test, identified commercially as the Covid antigen test, uses a concept of immunoassay. This assay captures specific antibody-antigen interaction and labels it using a conjugate—another molecule that enhances the signal detection. Though a distinct feature of GlycoGrip lies in exploiting glycan-antigen interaction, this device still needs a bioconjugate for its detection to work. This brought the authors to screen for different antibodies labeled with gold nanoparticles and see which molecules co-bind the virus’ spike effectively when GAG is bound to the surface. Marked by the most intense signal generated in under 5 minutes incubation time, the authors chose a gold-paired antibody for the N terminal domain (AuNP-NTD Ab), a surface that sits behind the connecting ridge of the spike in between the space occupied by GAG.
Before packing everything in a test strip, Dr. Ronit Freeman (a corresponding author of the study) and team optimized the best GAG molecules to bind the virus. By using strips made from nitrocellulose membrane, they planted the bioconjugate from the previous experiment and made the testing conditions uniform except for the GAG components. Again, by looking at the color intensity produced in control and test lines, two different components Heparan Sulfate (HS) and Heparin (HEP) showed the most intense red colors produced from nanoparticle reactions. To choose which one is the most suitable to be mass-produced commercially, Dr. Freeman considered the fees of synthesizing both molecules and decided to choose HEP as its surface-anchored GAG.
One disadvantage of using GAG for detection is its selectivity falls short compared to proteins. To find out if that were the case, Dr. Freeman mixed betacoronavirus spike glycoproteins with proteins usually found in human samples such as ACE2 and albumin. These mixtures, along with a pure SARS-CoV-2 spike, were dropped on the sample pad of GlycoGrip devices and compared for its signal generation. Pure SARS-CoV-2 spikes were one of the substances that generated the red signal, while mixtures of biological distractors and other viruses of the genus betacoronavirus did not. A mixture of SARS-CoV-2 spikes with distractors generated the same signal, but with the same or lesser intensity compared to the pure substances, suggesting GlycoGrip minimizes the possibility of false positive results.
Attested by this, the researchers tried to apply the same method clinically using human saliva samples. This was done because human samples are more complex, with many distractors present in one drop of human saliva. Different concentrations of SARS-CoV-2 spike were added to the saliva and tested. The limit of detecting the spike was very small, and again corroborates the result from the previous experiment that the reaction between HEP and the viral spike is specific enough.
With the emergence of SARS-CoV-2 variants in late 2020, the researchers were curious whether or not GlycoGrip can capture them as effectively as it did on wild type. Through a computational model and experiment on the strips, no specific mutations for variants of concern (VOC) Alpha, Beta, and Delta interfered with GAG binding. This sugar-coated strip is on course to be used as another point-of-care test (POCT) with Dr. Freeman and team currently testing the device against the Omicron variant. “We are optimistic that GlycoGrip will capture future variants just as easily,” she mused.
REFERENCES
Kim, Sang Hoon, Kearns, Fiona L., Rosenfield, Mia A., Casalino, Lorenzo, Papanikolas, Micah J., Simmerling, Carlos, Amaro, Rommie E., & Freeman, Ronit. (2021). GlycoGrip: Cell Surface-Inspired Universal Sensor for Betacoronaviruses. ACS Central Science, 8(1), 22-42, DOI: 10.1021/acscentsci.1c01080
COVID-19 gets its just desserts with sugar-coated test strips. (December 2021). Available: https://college.unc.edu/2021/12/covid-test-strips-freeman/ [Accessed: 25 January 2022]