Aleksandra Czezyk, Charlotte Burn, Claire Russell
Royal Veterinary College, University of London 4 Royal College St, London NW1 0TU, United Kingdom
ABSTRACT
There are extensive studies investigating the effects of enrichment on the welfare and behaviour of commonly used laboratory animals. However, when it comes to zebrafish, Danio rerio, there is limited knowledge available regarding what type of enrichment is beneficial to this species. More evidence needs to be collected to determine if providing refugia has a significant effect on reducing aggressive behaviours seen in laboratory zebrafish, typically housed in groups of 20 - 49. In this study, we observed the frequency of aggressive behaviours of zebrafish in their home tanks when the tanks were barren and enriched with a pup tent, lily pad or artificial plant. The frequency of enrichment use was also recorded. Then, a preference test was performed to see which enrichment the fish would select when given the choice. A significant reduction in aggression was seen with the pup tent and artificial plant (p = 0.0036 and p = 0.0047 respectively). When given the choice, zebrafish had a significant preference for the artificial plant and lily pad (p < 0.0001 for both). This study also revealed that enrichment had no negative effects on water quality when tested over a period of a week. In conclusion, the results indicate that providing refugia reduces the frequency of aggressive behaviours in zebrafish and that their choices are influenced by social context within home tanks. This knowledge can be used to enhance laboratory conditions and to ensure that humane and ethical methods are used in scientific research worldwide to promote animal welfare.
INTRODUCTION
Background
A large proportion of the current knowledge surrounding human biology, physiology, endocrinology, and pharmacology has been acquired by studying mechanisms in several different animal models (Hau, 2008). In comparison to humans, most animals have a shorter generation time and a reduced genetic variation (Zabel and Klose, 2008). In the UK, fish have become the second most used model in experimental procedures. Zebrafish, Danio rerio, are the most commonly used as they have many important attributes when compared to other model animals (White et al., 2017; Graham et al, 2018). For example, in contrast to conventional models such as Drosophila melanogaster or Caenorhabditis elegans, Danio rerio are vertebrates and thus are more physiologically related to humans; their internal mechanisms are relevant to human pathogenesis and clinical treatments (Egan et al., 2009). For example, zebrafish are a reliable model for anxiolytic drug screening because their primary stress hormone is cortisol (same as humans), not corticosterone. Egan et al. (2009) examined zebrafish behavioural responses to a selection of anxiolytic (e.g. ethanol) and anxiogenic (e.g. caffeine) factors. The results could be translated to treat stress and affective disorders in humans by testing new potential psychotropic drugs (Stewart et al., 2012).
Moreover, in comparison to other vertebrate models such as rodents, zebrafish develop faster, are smaller in size, have a faster breeding cycle, and transparent embryos. This makes them highly desirable for scientific research including developmental, behavioural, genetic, and disease studies. Furthermore, zebrafish are inexpensive and low-maintenance species which allows them to be easily kept in large numbers in relatively small areas, thus making them ideal models for large-scale biomedical research (Flinn et al., 2008; Egan et al., 2009; Oliveira, et al., 2011; White et al, 2017; Graham et al, 2018).
Wild vs laboratory conditions
As the prevalence of zebrafish in animal research continues to increase, there is a pressing obligation for the development of maintenance recommendations that are species-specific and will enhance zebrafish well-being, as well as improve research quality and efficacy. Zebrafish are frequently encountered in distinctive geographic regions with diverse habitats and climates, including India, Bangladesh and Nepal (Liss et al., 2015). Zebrafish reside in floodplain habitats with profuse vegetation where they occupy the upper to middle zone of standing or slow-moving water bodies such as rice paddles or man-made farm ponds. Zebrafish associate with each other in small, mixed sex groups consisting of 5 - 20 individuals (Harper and Lawrence, 2011; Liss et al., 2015). It has been found that they can be very territorial and compete for resources, including food and mates. Zebrafish display aggressive behaviours between and within sexes; these are driven by reproductive impulses, and thus arise when fish become sexually mature within several months after hatching. However, once dominant hierarchies are established within the group the frequency and intensity of aggression decreases (Spence et al., 2008; Paull et al., 2010; Schroeder et al., 2014; Graham et al., 2018).
Laboratory zebrafish welfare is significantly affected by housing densities; stress hormones like cortisol tend to spike in high and low densities (Liss et al., 2015). Furthermore, while adult individuals will naturally defend their territories, its intensity and frequency rise in crowded conditions, leading to increased aggression. Therefore, it is recommended that zebrafish are housed at low holding densities of maximum 5 fish per litre (Spence et al., 2008; Harper and Lawrence, 2011). In their natural habitats, zebrafish utilise plants for protection from predators, especially during spawning and oviposition (Spence et al., 2007; Liss et al, 2015). A study performed by Kistler et al. (2011) explored the influence of environmental complexity on zebrafish behaviour and preference, its results displayed a preference for structured environments when zebrafish were given the choice.
Although zebrafish laboratory housing conditions differ greatly from their natural habitats, the aforementioned biological factors must be taken into consideration when designing laboratory enclosures for zebrafish. To prevent experimental results being affected by husbandry changes, and to ensure fish health and productivity, they should be housed in water of consistent and specific range of parameters (Table 1). As water quality is key for keeping zebrafish in a salubrious aquatic environment; to maintain this, zebrafish should be kept in a continuously circulating filter system that aerates the water, and fish tanks should be cleaned at regular intervals (Advesh et al., 2012; Liss et al., 2015).
Welfare issues and enrichment
At present there is limited information regarding zebrafish welfare, social motivation and behaviour. The divergence from their natural habitat, as well as simplification of their environment, may result in inappropriate social contexts including overcrowding or isolation, thus restricting the range of social dynamics of captive zebrafish. Assessing zebrafish welfare is a complex process as it needs to consider physical and psychological health of the captive animal, as well as satisfying the five needs described in the 2006 Animal Welfare Act: 1) need for suitable environment, 2) need for suitable diet, 3) need to be able to express normal behaviours, 4) need to be housed with, or apart from, other animals, 5) need to be protected from pain, suffering, injury and disease (United Kingdom, Department for Environment, Food & Rural Affairs, 2013). Practical changes of laboratory conditions may enhance animal welfare; however, they may also negatively influence experimental results. Most zebrafish bred and reared in captivity are housed in barren tanks with limited added environmental complexity. Though in 2005, the European convention no. 123 for the protection of vertebrates used in research has stated that environmental enrichment (EE) improves welfare and should be applied to all captive animals. EE relates to any modification of a captive animals’ husbandry, including structural enrichment, in addition to social context. It aims to improve the animal’s biological functioning by creating a more naturalistic environment which satisfies animal’s behavioural needs; for example, laboratory zebrafish are fed live brineshrimp prey as it simulates their natural predator behaviours. Captive fish are also presented with marbles or images of pebbles to induce spawning. Unfortunately, there is limited knowledge as to what other types of enrichment are valuable to zebrafish. In addition, there is conflicting evidence about whether providing enrichments, such as refugia, plants or substrate has a significant effect on zebrafish aggressive and socio-positive behaviours (Newberry, 1995; Spence et al., 2008; Schroeder et al., 2014; Graham et al., 2018).
There is ample evidence suggesting the long-term benefits of a more complex and enriched laboratory enclosure has on zebrafish samples. A study performed on zebrafish locomotor activity investigating stress-related behaviours showed that individuals seek protection in novel environments (Egan et al., 2009). Furthermore, zebrafish are active and shoaling species, but studies have shown that they spend significantly more time in enriched tank compartments compared to barren ones (Spence et al., 2008; Liss et al., 2015; Graham et al., 2018). Based on this, it can be assumed that provision of hiding spaces in laboratory conditions would be beneficial as it has the potential to provide a sense of security, and as a result reduce zebrafish stress responses e.g. exploration and erratic movements (Baumans, 2005; Spence et al, 2008; Egan et al, 2009; Schroeder et al, 2014).
Other studies have shown that there may be detrimental effects to more structural or tank enrichment. A study performed by Schroeder et al. (2014), investigated the preference of zebrafish, housed in pairs and small groups, for a range of enrichments. Tanks were divided into two compartments containing different enrichments including: substrates, artificial plants, combinations thereof, and air stones. Preference was determined by comparing the time spent in enriched versus barren compartments. Results demonstrated that zebrafish favoured structural enrichment over standard conditions. Additionally, in pairs, the dominant fish would behaviourally exclude the subordinate from the substrate. This highlights the issues of using enrichment as it may lead to increased competition, as well as terrorisation of zebrafish at the bottom of the hierarchy (Schroeder et al., 2014; White et al., 2017). Moreover, using enrichment increases the risk of compromising water quality which may have deleterious effects on fish health and productivity. Standardised environmental conditions have been designed with the aim of increasing reproducibility of the results and reducing individual differences within the group (Baumans and Loo, 2013; Liss et al., 2015; Graham et al., 2018).
Measuring animal behaviour and welfare
Defining animal welfare has been a major difficulty throughout scientific literature. Some researchers equate animal welfare with biological fitness, suggesting that poor welfare only occurs if the animal’s survival and reproduction ability is hindered (Barnett and Hemsworth, 1990; Mason and Mendl, 1993). In comparison, other researches claim that animal welfare is impaired if the animal is experiencing an unpleasant mental state, even in the absence of physical problems (Dawkins, 1990; Mason and Mendl, 1993). This latter definition is applied to this study because although the zebrafish subjects are “healthy”, increased stressors from unpleasant mental states and aggressive dominant fish can have longer-term effects. There are some specific physiological and behavioural changes that clearly indicate poor animal welfare and are agreed upon by researchers worldwide. These include gastric ulceration, poor immune system function, reproductive problems such as reduced fertility, and behavioural problems such as extreme apathy, stereotypy, and even infanticide (Mason and Mendl, 1993). There are three main challenges associated with assessing animal welfare. First, different measures can yield results that do not always co-vary, whilst some indicate that welfare has reduced, others may indicate it has not changed, or even improved. Second, interpreting the significance of some measures is challenging as results may differ depending on the situation. Thirdly, even if a study yields an unambiguous conclusion, a repeat of that study can give rise to a contradictory result, possibly due to the subtly different conditions or characteristics of the animal (Dawkins, 1980; Barnett and Hemsworth, 1990; Dawkins, 1990; Sandoe and Simonsen, 1992; Mason and Mendl, 1993).
Hypothesis and aims
The aim of the study is to determine if introducing hiding spaces into tanks will significantly affect aggressive behaviour without compromising the water quality. To test this, two tanks will be introduced, each containing a different type of enrichment, or no enrichment, and the frequency of aggressive behaviours will be recorded. Then, a preference test will be carried out to determine if fish have a preference when given the choice.
Preference tests are used to study behavioural changes observed after animals’ living conditions are altered; they are highly advantageous as they give animals an opportunity to express their natural preferences. A great advantage of preference testing is that it can assess the strength of preferences for a certain stimulus by comparing them against a second, well understood stimulus e.g. food. Preference tests provide valuable information on animals’ reactions to husbandry, handling, housing, food, EE; this can be utilised to improve different aspects of animal living in captivity (Fraser and Matthews, 1997). The enhancement of laboratory conditions ensures humane and ethical methods are used in scientific research; this increases the validity and reliability of experimental data collected.
The hypothesis is that provision of hiding spaces will reduce the frequency of aggressive behaviours of zebrafish housed in a large group.
METHODS
Subjects and maintenance
This study was carried out under Home Office Project licence PPL PEB686695. Two tanks of 26 adult zebrafish were assessed, each tank contained 13 males and 13 females. The fish in the tanks were genetically modified; the line name of fish in tank 1 was cln6a12CAINS; the mutated gene is predicted to be involved in lysosome organisation (ZFIN, 2004). The line name of fish in tank 2 was tpp1sa0011; Tg (ELAV: GCaMP6s); this is a point mutation which leads to a premature stop codon. Mutant fish have a smaller retina and head, and a curved body (Mahmood, et al., 2013). However, no fish were homozygous so all fish were essentially normal.
Prior to, and during the experiment, these fish were held in barren tanks with a maximum stocking density of 49 adult zebrafish and water volume of 9800 ml3. Furthermore, the animals were fed 3 times a day at 10 AM, 12 PM and 4 PM; their food included Hikari pellet, brine shrimp and krill. Their photoperiod cycle was continuously controlled; the lights were turned on at 9 AM and off at 11 PM daily. Throughout the experiment, the water pH was maintained between 6.08 and 7.84, whilst the water temperature was maintained between 26.3 and 28.4˚C. During this study one fish in tank 1 had to be culled due to being egg bound.
Figure 2. Different types of enrichment used in this experiment. A = artificial plant, B = pup tent, C = lily pad.
Enrichment
The structural enrichment chosen for this experiment was based on the features of a natural zebrafish habitat, including an artificial plant, a plastic pup tent, and a plastic lily pad (Figure 2). The artificial plant was chosen because it resembled aquatic vegetation often found in a zebrafish habitat and has been used in previous studies (Kistler et al., 2011; Schroeder et al., 2014). Also it was made out of flexible, soft PVC so it would not injure the fish. The plastic lily pad was thought to imitate protection from above predators in a natural zebrafish environment. Lastly, the semi-opaque pup tent was chosen to provide shelter and security from other aggressive group members. (Datesand, Datesand: Our products/ Aquatic Enrichment, 2016).
Experimental procedure
Home tank assessment
The behaviour of zebrafish was assessed by video recording over a period of six weeks. The fish were filmed twice a day for 10 minutes, in the morning at 11AM ± 2 hours and in afternoon at 2PM ± 2 hours. Every Friday afternoon, the enrichment was changed. This gave the fish time over the weekend to acclimate to the environmental change (Table 2). Each enrichment was assessed for a week. Additionally, a barren environment was assessed and acted as a control.
The fish were observed instantaneously for 10 seconds at 1-minute intervals. Data were collected on the frequency of aggressive behaviours and the number of times the fish use the enrichment by swimming through or underneath it. Additionally, the number of times the enrichment was used was recorded; this included situations when a fish would swim through or underneath the enrichment whilst being chased by another fish (see Appendix for recording sheet). An ethogram of behaviours, derived from Kalueff et al. (2013), was created to specify aggressive behaviours and help distinguish them from normal zebrafish behaviours (Table 3).
Figure 3. Photograph showing the set-up of the cross maze used for preference testing of different types of enrichment. A = artificial plant, B = Pup tent, C = Lily pad, D = Barren.
Preference test
Five fish were selected at random from each tank and were assessed using a cross maze preference test, one at a time. A fish was placed in the cross maze and was given a 30-minute acclimation and recovery period from handling stress. Each arm of the cross maze had a different type of enrichment or no enrichment (Figure 3). After 30 minutes, the fish was filmed for a 10-minute period. This process was repeated 3 times per fish. Data was collected on the duration of time spent in each section of the cross maze (see Appendix for recording sheet).
Water quality tests
Prior to the experiment, fresh tank water was tested using NTLABS Aquarium Lab – multi-test water quality test, the key parameters evaluated were: ammonia, nitrite, nitrate, pH, alkalinity and general hardness. At the end of each week the water in the tanks was tested to check if the enrichment had any negative effects on the quality (Figure 4). These results were then compared to those obtained from the water tested at the beginning of the study.
Figure 4a-b. Photographs showing the NTLABS Aquarium Lab. Multi-test water quality test being carried out, including the instructions (NTLABS, 2018).
Statistical analysis
Home tank assessment
First, a graph was created to represent the activity levels of fish in each tank; it compared the mean values + standard deviation of the control and the enriched tank for the frequency of aggression, use of enrichment, and use of enrichment to avoid aggression. Thence, separate graphs were created to demonstrate the changes in frequency of aggression within each week for each type of enrichment.
Then, the data collected from each tank was analysed separately using GraphPad Prism 7.01 for Windows. The data collected from the home tank assessment for the frequency of aggression was grouped into a table where one group represented the control tank (column factor) and one group represented the enriched tank, and each row represented a different time point (morning or afternoon); matched values were stacked into a subcolumn. A two-way ANOVA was conducted to determine if there was a significant difference in the frequency of aggression between column factor and / or time factor.
Next, the data for the frequency of aggression from the two tanks was combined for each type of enrichment. It was put into a table with groups A, B, C, and D which represented the enriched tank in the morning, enriched tank in the afternoon, control tank in the morning, and the control tank in the afternoon, respectively. The rows represented day 1 - 5. A three-way ANOVA was conducted to determine if there was a significant difference in the frequency of aggression between the enriched and control tanks, as well as between the time of the recordings.
Thereafter, three separate tables were made for the frequency of aggression, enrichment use, and coincidental use of enrichment for tank 1 and tank 2 combined. The data were grouped into tank 1 and tank 2, and the rows represented the type of enrichment or the control. Each set of data was then analysed using a two-way ANOVA multiple comparisons test, which compared each cell mean with the other cell mean in that row; this determined if there was a significant difference in aggression, enrichment use, and coincidental use of enrichment between tank 1 and tank 2 and compared each type of enrichment to the control.
Preference test
The data collected from the preference test was grouped into a table where each group represented each fish that was tested, each column represented a different time point (duration of time (s) spent in each cross-maze section), and each row represented a different type of enrichment (row factor). The data was analysed, using a two-way ANOVA multiple comparison test (comparing column means) to determine if there was a significant difference between row factor and/or time factor; first this was done for tank 1, then for tank 2, and lastly for the tanks combined. Then a graph was created per tank and for the tanks combined, to represent the mean amount of time each fish spent in each section of the cross maze.
Water quality tests
Water quality test results for each parameter were put into one graph which compared the values obtained from the control and enriched tanks; this determined if the enrichment had a negative impact on the water quality.
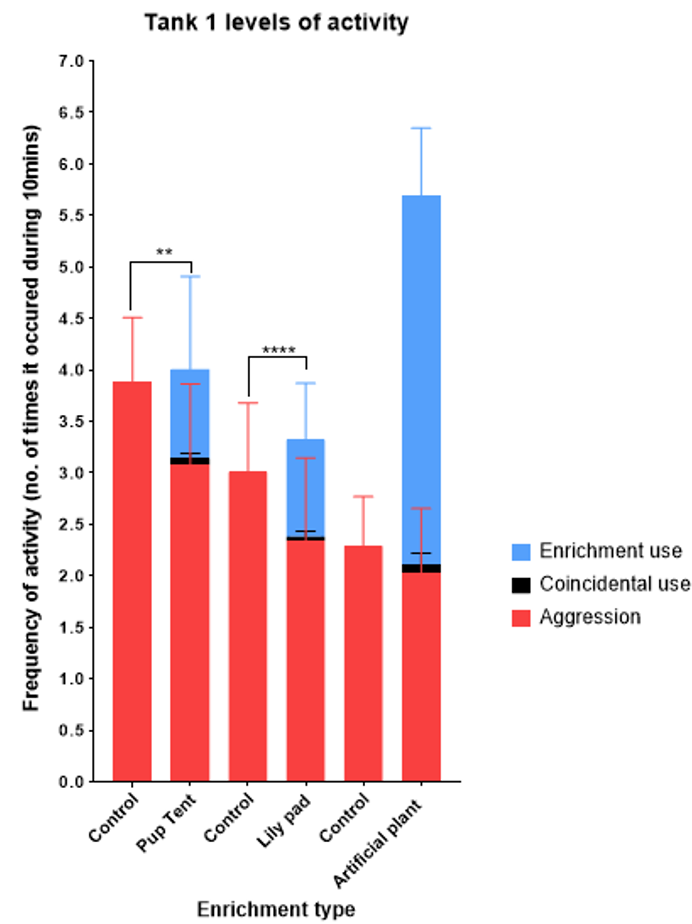
Figure 5. Frequency of fish activity in tank 1 between the different types of enrichment compared to control environment. Values are mean + SD. Significant differences are indicated by a ** (p < 0.01) and **** (p < 0.0001).
RESULTS
Home tank assessment
Tank 1
The graph (Figure 5) demonstrates that the frequency of aggression (number of times it occurred during 10min) decreased when each enrichment was introduced into the tank. The frequency of aggressive behaviours was the lowest when the tank was enriched with the artificial plant (2.02), however the greatest reduction in frequency of aggression was seen with the pup tent (30.45% less than the control), compared to the lily pad (22.30% less than the control) and the artificial plant (7.86% less than the control). The most frequently used enrichment was the artificial plant (3.58), the lily pad and the pup tent were used considerably less frequently (0.94 and 0.87 respectively). The coincidental use of enrichment also had the highest frequency for the artificial plant (0.09), compared to the pup tent (0.06) and the lily pad (0.04).
Graphs representing weekly changes in the frequency of aggression (Figure 6a-c) have shown that the enriched tank had a lower frequency of aggression compared to the barren tank. In week 2, the pup tent vs barren tank was assessed; the average frequency of aggression ranged from 1.7 - 4.2 and 2.8 - 4.8 respectively. Figure 6a demonstrates that the average frequency of aggression was lower in the afternoon when the tank was enriched with the pup tent, whereas in the barren tank the time of day did not affect aggression frequency. In week 4, the lily pad vs barren tank was assessed; Figure 6b shows that the average frequency of aggression ranged from 1.1 - 3.4 with the lily pad, and 2.1 - 4.1 without it. Figure 8b also showed that the fish were on average less aggressive in the afternoon when the lily pad was present, whereas the time of day did not affect frequency of aggression in the barren tank. In week 6, the artificial plant vs barren tank was assessed; the average frequency of aggression ranged from 1.1 - 3.4 when the tank was enriched, and 1.6 - 3.1 when the tank was barren. Figure 6c again shows that the average frequency of aggression was lower in the afternoon when the tank was enriched with the artificial plant, and lower in the morning when the tank had no enrichment.
Statistical analysis on data collected from tank 1 showed a significant decrease in the frequency of aggression when the tank was enriched with the pup tent (p = 0.004) and the lily pad (p = <0.0001), compared to the control environment. However, there was no significant decrease in the frequency of aggression when the tank was enriched with the artificial plant (p = 0.06). Results have also shown that the frequency of aggression was significantly lower in the afternoon than in the morning, only when the tank was enriched with the lily pad (p = 0.003).
Tank 2
The graph (Figure 5) demonstrates that the frequency of aggression decreased with the artificial plant (18.04% less than the control) and the pup tent (4.63% less than the control) but increased with the lily pad (10.14% greater than the control). The frequency of aggression was the lowest when the tank was enriched with the artificial plant (4.09) and the highest with the pup tent (5.15). The most frequently used enrichment was also the artificial plant (4.87), and the least frequently used enrichment was the pup tent (1.01). The coincidental use of enrichment was the highest for the pup tent (0.31) and the lowest for the lily pad (0.14).
Figure 6a-c. Tank 1 weekly changes in the frequency of aggression when different types of enrichment were introduced, compared to a control environment. Includes data from morning and afternoon recordings. Values are mean + SEM.
Graphs representing weekly changes in the frequency of aggression (Figure 6a-c) have shown that the average frequency of aggression was lower when the tank was enriched with the pup tent and the artificial plant, however it was higher when the tank was enriched with the lily pad, compared to when the tank was barren. In week 1, the average frequency of aggression ranged from 3 - 9 when the tank was enriched with the pup tent, whereas the barren tank ranged from 3.4 - 7.2. Figure 6a demonstrates that the average frequency of aggression was not affected by the time of day when the pup tent was present, however without enrichment the frequency of aggression was lower in the morning. In week 3, Figure 6b shows that the average frequency of aggression ranged from 3.9 - 5.7 with the lily pad, and 3.5 - 5.7 with no enrichment. Figure 6b showed that the frequency of aggression was lower in the afternoon with and without the enrichment. In week 6, the artificial plant vs barren tank was assessed, the average frequency of aggression ranged from 2.9 - 5.3 when the tank was enriched, and 3.4 -- 7.6 when the tank was barren. Figure 6c shows that the average frequency of aggression was lower in the morning with the enrichment, and lower in the afternoon without it.
Results from the statistical analysis on data collected from tank 2 showed there was a significant decrease in the frequency of aggression when the tank was enriched with the artificial plant (p = 0.005), however there was no significant difference for the pup tent (p = 0.414) and the lily pad (p = 0.070) when compared to the control tank. Results have also demonstrated that there was a significant decrease in the frequency of aggression during the afternoon recordings compared to the morning recordings with the pup tent (p < 0.001), the lily pad (p = 0.02), and the artificial plant (p = 0.006).
Combined results
The results of the three-way ANOVA test, performed on the data combined from both tanks, demonstrated that the pup tent and the artificial plant significantly decreased the frequency of aggression in the home tanks (p = 0.014 and p = 0.010 respectively), whereas the lily pad had no significant difference (p = 0.667). Statistical analysis also concluded that there was no significant difference in the frequency of aggression for the time of the day, for any of the enrichments.
When comparing data from the tanks, the statistics show that the fish in tank 2 were, on average, significantly more aggressive than the fish in tank 1 (4.94 > 3.06); this was true for when the tanks were enriched or barren (p < 0.0001). Furthermore, the average use of enrichment by fish in tank 2 occurred more frequently than in tank 1 (2.40 > 1.80); however, the difference was significant only when the artificial plant was present (p < 0.001). Additionally, on average, the coincidental use of enrichment also occurred more frequently in tank 2 than in tank 1 (0.23 > 0.06); although the difference was only significant for the pup tent (p < 0.0001) and the artificial plant (p = 0.0056).
Figure 7a-b. The average time spent by each fish in each section that contained a different type of enrichment or no enrichment. Values are mean + 95% CI. Significant differences (p < 0.001; 2way ANOVA multiple comparison test) are indicated by a (****).
Preference test
Tank 1
The graph (Figure 7a) demonstrates that the five random fish selected from tank 1 spent the most time in the cross-maze section that contained the artificial plant (1660s); they spent less time in the section that contained the lily pad (685s) and the pup tent (424s). The least amount of time was spent in the barren section of the cross maze (200s). The two-way ANOVA multiple comparison test determined that there was a significant difference in the time spent by the fish in the section containing the artificial plant when comparing: artificial plant vs lily pad (p < 0.0001), artificial plant vs pup tent (p < 0.001), and artificial plant vs barren section (p < 0.0001).
Tank 2
The graph (Figure 7b) demonstrates that the five random fish selected from tank 2 spent the most time in the cross-maze section that contained the lily pad (1674 s); they spent less time in the sections that contained the pup tent and the lily pad (474 s and 473 s respectively). The least amount of time was spent in the barren section of the cross maze (261 s). The two-way ANOVA multiple comparison test determined that there was a significant difference in the time spent by the fish in the section containing the lily pad when comparing: lily pad vs artificial plant (p < 0.0001), lily pad vs pup tent (p < 0.0001), and lily pad vs barren (p < 0.0001).
Combined results
When the preference test results for all 10 fish from both tanks were combined (Figure 12), they demonstrated that the fish spent the most time in the section that contained the lily pad (2359 s), then the artificial plant (2133 s), then the pup tent (899 s); the least amount of time was spent in the barren section (461 s). Statistical analysis using a two-way ANOVA multicomparison test determined that the fish spent significantly less time near the pup tent than near the lily pad and the artificial plant (p < 0.0001). There was also a significant decrease in the time spent in the barren section compared to the section containing the lily pad and the artificial plant (p < 0.0001).
Water quality test
The results obtained from the water quality tests showed little variation between the values of the enriched and control tank water. The recommended parameter values suggested by the manufacturer of the water quality test were as follows: NH2 0 mg/L, NO2 0 mg/L, NO3 0 mg/L, pH 7/8, KH 6°dH, GH 8°dH; only the pH remained within the recommended value throughout (Table 4).
DISCUSSION
This study evaluated zebrafish behaviour by introducing three different types of environmental enrichment into tanks to determine if they would reduce the levels of aggression of zebrafish housed in a large group. Then, a preference test was performed to assess which, if any, type of enrichment was preferred by the zebrafish. Water quality was monitored weekly to determine if the enrichment had any negative effects on key chemical and physical parameters.
Home tank assessment
The results obtained from each tank support the hypothesis that adding hiding spaces significantly reduces zebrafish aggression. In addition, they showed that fish in tank 1 were on average less aggressive than fish in tank 2, which could have been the cause of dissimilar results in enrichment use. Studies have shown that there are significant differences between strains of zebrafish in most behaviour patterns, including but not limited to: shoaling, activity levels, predator approaches, latency to feed after disturbance, and biting to a mirror stimulus (Moretz et al., 2007). Fish in tank 1 had a mutation in the cln6a gene and fish in tank 2 had a mutation in the tpp1 gene; the different genetic modification could have been the reason for the differences seen between the tanks. This demonstrates that researchers should carefully consider behavioural differences when using various strains of zebrafish (Egan et al., 2009). Fish in tank 1 were the least aggressive when the pup tent was present, whereas fish in tank 2 were the least aggressive when the artificial plant was present. The more aggressive fish in tank 2 used the enrichment more frequently, including coincidental use whilst being chased by another fish. This suggests a correlation between the frequency of enrichment use and the levels of aggression.
Within each tank there was variation in the frequency of aggression linked to the time of day. Fish in both tanks were more aggressive in the morning; this could be because zebrafish express a diurnal activity pattern (Hurd et al., 1998). In laboratory zebrafish, spawning behaviour is influenced by the photoperiod and the feeding cycle (Spence et al., 2008). The first spawning activity often occurs within the first minute of light exposure and continues for about an hour. Zebrafish courtship behaviour is characterised by the male chasing and circling the female rapidly, which resembles aggressive behaviours (Gerlach, 2006; Hansak et al., 2010). A study by Spence et al., (2008) examining zebrafish behaviour has shown that aggression rates during spawning are higher at higher stocking densities. However, combined data from this experiment showed no significant difference between the morning and the afternoon. During this experiment, the fish were filmed between 9:30 AM and 12 PM - after being fed and after morning spawning.
Preference test
The fish from tank 2 had a significant preference for the lily pad, however the fish from tank 1 preferred the artificial plant. These results agree with the previous ones as aggression levels were reduced significantly by the artificial plant in the home tank (see Figure 5). Natural zebrafish habitats are associated with the presence of aquatic vegetation. This could be the reason for the exhibited preference of the artificial plant. A similar study performed by Delaney et al., (2002) found that zebrafish spent 99% of time in the area containing artificial plants, supporting the results found in this experiment. However, additional considerations should be made. In the wild, zebrafish are targeted by predators such as snakeheads or fresh-water garfish; the structure of the cross maze used for the preference testing had an open top. This exposed the fish to the surroundings which in the wild would have increased their perceived risk of predation (Spence et al., 2008). Therefore, the preference for the lily pad observed from the combined data could have been linked to seeking safety and protection from above.
Another study, performed by Schroeder et al., (2014), determined the preferences of zebrafish, housed in pairs and groups, by exposing them to a range of enrichment cues. The cues were chosen to resemble features often seen in natural zebrafish environments, including substrates, artificial plants, and air stones, yet they were still preferred by zebrafish which were bred and reared in barren laboratory conditions. Zebrafish housed in groups showed the most significant preference for gravel substrate, either as an individual cue or in combination with the artificial plant; this choice was influenced by gender. In comparison, the preference of paired zebrafish was influenced by dominance status as dominant / subordinate relationships being formed in all pairs; the results showed that dominant fish also expressed a significant preference for gravel substrate and the frequency of aggressive behaviours increased in pairs (Dahlbom et al., 2012). This implies that the preference for a certain type of enrichment exhibited in this current study could have been influenced by the social dynamics within the tanks, i.e. dominance and hierarchy.
The findings reported in this study are also supported by a previous study carried out by Collymore et al., (2015) which also found that zebrafish prefer a complex environment to a barren one. They recommended introducing plants into the tanks as the plants provided protection from aggressive members of the group, which in return enhances zebrafish wellbeing. However, it could be argued that provision of a single enrichment might not be sufficient because the dominant fish exclude the subordinate fish from using the preferred enrichment, leaving subordinates without access to such hiding locations (Schroeder et al., 2014). A study performed by Woodward et al., (2019) showed that fish housed in very enriched tanks became more aggressive over time. Additionally, as described in the methods, the number of fishes in the tanks was rearranged to get 13 of each sex. This could have disrupted the previously established hierarchy and affected the aggression levels.
A further study was carried out by Schroeder et al. (2014) to investigate the neural consequences that enrichment has on zebrafish. The results have shown that there was an increased cell proliferation in the forebrain of zebrafish kept in tanks enriched with gravel and artificial plants than in fish kept in barren tanks which suggests improved brain development. This further highlights the advantages of providing environmental enrichment in captive zebrafish and advocates that standard barren environments do not meet the needs of captive-bred zebrafish, thus leaving a potentially impaired nervous system function.
Similar findings have been recognised in other fish species including brown trout (Salmon trutta), three-spined stickleback (Gasterosteus aculeatus) and red snapper (Lutjanus campechanus) (Schroeder et al., 2014). This indicates that the provision of environmental enrichment, such as substrate and refugia, has beneficial effects on teleosts; it proposes that habitats with high structural complexity promote foraging behaviours and reduces boredom behaviours seen in barren environments (Schroeder et al., 2014).
Water quality tests
The most important factor in successful fish husbandry is the maintenance of good water quality. Water should be tested regularly to ensure that the chemical and physical parameters are at appropriate levels. Poor water chemistry will have a negative impact on the fish welfare and thus lead to stress. Increase in stress levels has a diminishing effect on fish health. It leads to a decreased immunity and makes them more susceptible to pathogenic infection (Westerfield, 1994; NTLABS, 2018).
During this study six key parameters were tested: ammonia, nitrite, nitrate, pH, KH (alkalinity) and GH (general hardness). Ammonia is a waste product made during normal fish metabolism, it is released via gills and excrement to avoid poisoning. Generally, in natural habitats the produced ammonia is diluted by the large volume of water. However, in a closed tank ammonia levels can rise quickly and become toxic which leads to distress amongst fish. Nitrite is produced when bacteria break down ammonia. High concentrations of this compound are dangerous to fish and cause nitrite poisoning. Nitrate is not as harmful to fish in lower quantities. However, when its concentration exceeds 80-100 mg/L, it may show signs of toxicity and lead to undesirable algae growth. In this study, the pH was regulated to ensure that the water is not too acidic or alkaline for the species living in it. Carbonates and bicarbonates are buffering agents present in the water, they act at a pH stabiliser, and so KH is a measure of carbonate hardness. GH is a measure of calcium and magnesium in the water which determines if the water is hard or soft (NTLABS, 2018).
The water quality test results have shown that the parameter values remained relatively constant throughout the experiment, thus indicated that introducing enrichment into the tanks did not have a negative effect on the water quality. Although the tank contained the enrichment only for a week at a time and the tanks are normally cleaned once a month, researchers should still monitor water quality regularly when adding enrichment to tanks. The major issue discovered in this study was that the results given by the NTLABS Aquarium Lab – multi-test water quality test kit were not of the recommended value provided by the manufacturer. This could have been caused by the kit not being accurate enough, or by the fact that it was open for a prolonged period, which could have affected the activity of the reactants. However, most of the values were still within the recommended ranges mentioned in Table 1 (see Introduction, Wild vs. laboratory conditions).
Limitations
Measuring animal behaviour and interpreting animal welfare is difficult as it relies on subjective judgements which can be influenced by the nature of human concern for the animal that is being observed. Animal responses often differ not only between species, but also between individuals, and in some cases the response can change in a single individual over time. The difference in response to the same situation can be linked to the variance in age and sex amongst animals. This could be driven by prior differences in their underlying physiological and neurological systems. Some researchers suggest that the differences can be attributed to concepts such as individuality, temperament or behavioural style of the animal. Furthermore, relying on an animal’s behaviour to accurately indicate its preference for, or aversion to, a specific environment also has some limitations, the biggest of which is that restriction of preference testing does not allow for the complexity of the animal’s environmental preferences. The choice made by the animal at a certain time might not truly reflect what the animal would have preferred, or benefited from, in the long term (Barnett and Hemsworth, 1990; Mason and Mendl, 1993; Frasaer and Matthews, 1997).
To ensure that the collected data was consistent and precise, an interobserver reliability test could have been carried out; fish videos could have been watched by another person who was blind to the hypothesis (Blaser and Rosemberg, 2012). Blinding would have eliminated any potential bias and confirmed the reliability of results.
Implications for future research
Additional studies are needed to investigate how social context affects zebrafish preference of enrichment type. For this, multiple zebrafish should be preference tested at the same time (see Methods, Experimental procedure, Preference test); this would help determine if group housing influences the type of enrichment that is preferred by zebrafish. Also, zebrafish are a shoaling species. Thus, isolation may cause increased stress and alter their behavioural needs and environmental preferences (Collymore et al., 2015). Exploring group preferences can enhance our understanding of zebrafish behaviour and provide a clearer outlook on their enrichment choices. This knowledge can aid refinement in zebrafish laboratory housing (Schroeder et al., 2014).
Captive conditions may often be perceived as threatening for the animals, even though they are not inherently dangerous. For laboratory fish, these conditions include water quality, physical disturbances, stocking density and social environments. These conditions may cause the activation of the hypothalamic-pituitary-interregnal (HPI) axis, either intermittently, or continuously, for prolonged periods of time. The activity of the pituitary-adrenocortical system is mentioned frequently in animal welfare research and is reflected by the levels of corticosteroids circulating in the blood (Mason and Mendl, 1993; Branson, 2008). High levels of corticosteroids, such as cortisol, can result in chronic stress; long-term exposure to aversive stimuli and social stress result in increased adrenocortical activity, which is characterised by an amplified cortisol response. This response has the potential to become maladaptive and harmful for the animal. Further research could be carried out to determine if enrichment has any effect on zebrafish stress levels. This could be achieved by measuring cortisol levels in water and faeces. This is a non-invasive method of detection, however it would necessitate the loss of individual variation, as well as the requirement for known inputs and outputs of an enclosed volume of water (Mason and Mendl, 1993; Branson, 2008).
Conclusion
The purpose of enrichment is to simulate an environment that will elicit an animal’s normal behaviour patterns, therefore reducing issues associated with captivity. The most common problem with cage design is that it does not maximise the use of available space. This can be achieved by counteracting the deficiencies in size of the enclosure. An example of such an approach is increasing the psychological space of the enclosure. This can be accomplished by understanding which aspects of space are important to the species of interest. This includes the activities that the animal would utilise the additional space for, for example using the space to avoid other individuals, hide from predators, or to forage for food (Chamove, 1989).
Overall, this study concluded that providing enrichment significantly reduces the frequency of aggressive behaviours performed by captive-bred zebrafish. The most effective type of enrichment was different for each tank, suggesting that enrichment choice is influenced by social relationships established within the tank. The strain of zebrafish also could have affected the enrichment choice. However, the most frequently used enrichment was the artificial plant, this was true for both tanks and suggests that this type of enrichment might satisfy a certain behavioural need of zebrafish. The combined preference test results have shown that, when isolated, zebrafish preferred either the artificial plant or the lily pad; this could be due to provision of protection from above. This study also revealed that none of the enrichments had a negative effect on water quality over a period of a week. This finding provides evidence that experimental results will be unaffected by the inclusion of enrichments, and therefore should encourage researchers to provide enrichment for captive zebrafish. By understanding which features of naturalistic zebrafish environments, such as substrates, plants, and hiding spaces, enable satisfaction of their ethological needs, zebrafish natural behaviour and development can be promoted. This could improve laboratory housing conditions and captive zebrafish welfare by focusing on their naturalistic cognitive aspects and by providing learning and exploration opportunities (Schroeder et al., 2014).
ACKNOWLEDGMENTS
I would like to thank my project supervisor, Dr. Claire Russell, and Dr. Charlotte Burn for their excellent supervision and guidance throughout the project, and for providing such a fascinating topic of research. I would also like to express my gratitude to the Datesand company for providing the different types of enrichment used in this experiment. Further appreciation goes to the staff at the Biological Services Unit for providing the zebrafish investigated and looking after them daily.
REFERENCES
Avdesh, A., Chen, M., Martin-Iverson, M. T., Mondal, A., Ong, D., Rainey-Smith, S., Taddei, K., Lardelli, M., Groth, D. M., Verdile, G. and Martins, R. N. (2012). Regular Care and Maintenance of a Zebrafish (Danio rerio) Laboratory: An Introduction. Journal of Visualised Experiments 69: e4196, 1-8., available: doi: 10.3791/4196.
Barnett, J. L. and Hemsworth, P. H. (1990). The validity of physiological and behavioural. Applied Animal Behaviour Science 25, 177-187, available: doi: 10.1016/0168-1591(90)90079-S.
Baumans, V. (2004). Methods for evaluation of laboratory animal well-being. Alternatives to Laboratory Animals, 32(1), 161-162, available: doi: 10.1177/026119290403201s26.
Baumans, V. (2005). Science-based assessment of animal welfare: laboratory animals. Revue Scientifique Et Technique-Office International Des Epizooties, 24(2) , 503-514, available: doi: 10.20506/rst.24.2.1585.
Baumans, V. and Van Loo, P. L. P. (2013). How to improve housing conditions of laboratory animals: the possibilities of environmental refinement. The Veterinary Journal, 195(1), 24-32, available: doi: 10.1016/j.tvjl.2012.09.023.
Blaser, R. E. and Rosemberg, D. B. (2012). Measures of Anxiety in Zebrafish (Danio rerio): Dissociation of Black/White Preference and Novel Tank Test. PLOS ONE, 7(5): e36931, 1-8, available: doi: 10.1371/journal.pone.0036931.
Branson, E. J. (2008). Fish Welfare. Blackwell Publishing Ltd: Oxford, UK, available: https://awionline.org/lab-animal-search/branson-e-j-ed-2008-fish-welfare-blackwell-publishing-ltd-oxford-uk-300-p.
Chamove, A.S. (1989). Environmental enrichment : a review, Animal Technology, 40(3), 155–178, available: https://ci.nii.ac.jp/naid/10016054675.
Collymore, C., Tolwani, R. J. and Rasmussen, S. (2015). The Behavioral Effects of Single Housing and Environmental Enrichment on Adult Zebrafish (Danio rerio). Journal of the Americal Association for Laboratory Animal Science, 54(3), 280-285, available: /pmc/articles/PMC4460940/?report=abstract.
Dahlbom, S. J., Backström, T., Lundstedt-Enkel, K. and Winberg, S. (2012). Aggression and monoamines: Effects of sex and social rank in zebrafish (Danio rerio). Behavioural Brain Research, 228(2), 333–338, available: doi: 10.1016/j.bbr.2011.12.011.
Datesand. (2016). Datesand: Our products/ Aquatic Enrichment, available: http://datesand.com/index.php/product/lilypad/ [accessed: 18 September 2019].
Datesand. (2016). Datesand: Our products/ Aquatic Enrichment, available: http://datesand.com/index.php/product/bamboo-plant/ [accessed: 18 September 2019]
Datesand. (2016). Datesand: Our products/ Aquatic Enrichment, available: http://datesand.com/index.php/product/mouse-tent-certified-aquatics/ [accessed: 18 September 2019]
Dawkins, M. (1980). Animal Suffering: The Science of Animal Welfare, Chapman and Hall; London, UK.
Dawkins, M. S. (1990). From an animal’s point of view: motivation, fitness, and animal welfare, Behavioral and Brain Sciences, Cambridge University Press, 13(1), 1–9, available: doi: 10.1017/S0140525X00077104.
Delaney, M., Follet, C., Ryan, N., Hanney, N., Lusk-Yablick, J. and Gerlach, G. (2002). Social interaction and distribution of female zebrafish (Danio rerio) in a large aquarium. The Biological Bulletin, 203(2), 240–241, available: doi: 10.2307/1543418.
Egan, R. J., Bergner, C. L., Hart, P. C., Cachat, J. M., Canavello, P. R., Elegante, M. F., Elkhayat, S. I., Bartels, B. K., Tien, A. K., Tien, D. H., Mohnot, S., Beeson, E., Glasgow, E., Amri, H., Zukowska, Z. and Kalueff, A. V. (2009). Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural Brain Research, 205(1), 38–44, available: doi: 10.1016/j.bbr.2009.06.022.
Flinn, L., Bretaud, S., Lo, C., Ingham, P. W. and Bandmann, O. (2008). Zebrafish as a new animal model for movement disorders. Journal of Neurochemistry, 106(5), 1991–1997, available: doi: 10.1111/j.1471-4159.2008.05463.x.
Fraser, A. F. (1983). The behaviour of maintenance and the intensive husbandry of cattle, sheep and pigs. Agriculture, Ecosystems and Environment, 9(1), 1–23, available: doi: 10.1016/0167-8809(83)90002-6.
Gerlach, G. (2006). Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Animal Behaviour, 72(5), 1119–1124, available: doi: 10.1016/j.anbehav.2006.03.009.
Graham, C., von Keyserlingk, M. A. G. and Franks, B. (2018) Zebrafish welfare: Natural history, social motivation and behaviour. Applied Animal Behaviour Science, 200, 13–22, available: doi: 10.1016/j.applanim.2017.11.005.
GraphPad Prism (2019). GraphPad Prism: Support, available https://www.graphpad.com/guides/prism/8/statistics/statwhentoplotsdvssem.htm [accessed: 23 April 2019].
Harper, C. and Lawrence, C. (2016). The Laboratory Zebrafish, CRC Press; USA, available: doi: 10.1201/b13588.
Hau, J. (2008). Animal models for human diseases: an overview, Source Book of Models for Biomedical Research, Humana Press, pp. 3–8, available: doi: 10.1007/978-1-59745-285-4_1.
Hurd, M. W., Debruyne, J., Straume, M. and Cahill, G. M. (1998). Circadian rhythms of locomotor activity in zebrafish. Physiology and Behavior, 65(3), 465–472, available: doi: 10.1016/S0031-9384(98)00183-8.
Hutter, S., Penn, D. J., Magee, S. and Zala, S. M. (2010). Reproductive behaviour of wild zebrafish (Danio rerio) in large tanks. Behaviour, 47(5–6), 641–660, available: doi: 10.1163/000579510X12632972473944.
Kalueff, A. V., Gebhardt, M., Stewart, A. M., Cachat, J. M., Brimmer, M., Chawla, J. S., Craddock, C., Kyzar, E. J., Roth, A., Landsman, S., Gaikwad, S., Robinson, K., Baatrup, E., Tierney, K., Shamchuk, A., Norton, W., Miller, N., Nicolson, T., Braubach, O., Gilman, C. P., Pittman, J., Rosemberg, D. B., Gerlai, R., Echevarria, D., Lamb, E., Neuhauss, S. C. F., Weng, W., Bally-Cuif, L. and Schneider, H. (2013). Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish, 10(1), 70–86, available: doi: 10.1089/zeb.2012.0861.
Kistler, C., Hegglin, D., Würbel, H. and König, B. (2011). Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Applied Animal Behaviour Science, 135(4), 318–327, available: doi: 10.1016/j.applanim.2011.10.014.
Liss, C., Litwak, K., Tilford, D., & Reinhardt, V. (2015). Zebrafish, Comfortable quarters for laboratory animals, 10th ed., Washington: Animal Welfare Institute, 88-98, available: https://awionline.org/sites/default/files/publication/digital_download/AWI-ComfortableQuarters-2015.pdf
Mahmood, F., Fu, S., Cooke, J., Wilson, S. W., Cooper, J. D. and Russell, C. (2013). A zebrafish model of CLN2 disease is deficient in tripeptidyl peptidase 1 and displays progressive neurodegeneration accompanied by a reduction in proliferation. Brain, 136(5), 1488–1507, available: doi: 10.1093/brain/awt043.
Martins, E. P. and Bhat, A. (2014). Population-level personalities in zebrafish: Aggression-boldness across but not within populations. Behavioral Ecology, 25(2), 368–373, available: doi: 10.1093/beheco/aru007.
Mason, G. J. and Mendl, M. (1993). Why is there no simple way of measuring animal welfare? Animal Welfare, 2, 301-319, available: https://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/10214/4673/Mason_&_Mendl_1993.pdf?sequence=1
Moretz, J. A., Martins, E. P. and Robison, B. D. (2007). Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behavioral Ecology, 18(3), 556–562, available: doi: 10.1093/beheco/arm011.
Newberry, R. C. (1995). Environmental enrichment: Increasing the biological relevance of captive environments. Applied Animal Behaviour Science, 44(2–4), 229–243, available: doi: 10.1016/0168-1591(95)00616-Z.
NTLABS. (2018). NTLABS: Knolwedge Hub, available: https://www.ntlabs.co.uk/knowledge-hub/water-quality-parameters-in-freshwater-aquariums/ [accessed 23 April 2019]
NTLABS. (2018). NTLABS: Products, available: https://www.ntlabs.co.uk/browse-products/indoor/aquarium-lab/aquarium-lab-multi-test/ [accessed 23 April 2019]
Oliveira, R. F., Silva, J. F. and Simões, J. M. (2011). Fighting zebrafish: Characterization of aggressive behavior and winner-loser effects. Zebrafish, 8(2), 73–81, available: doi: 10.1089/zeb.2011.0690.
Paull, G. C., Filby, A. L., Giddins, H. G., Coe, T. S., Hamilton, P. B. and Tyler, C. R. (2010). Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish, 7(1), 109–117, available: doi: 10.1089/zeb.2009.0618.
Sandøe, P. and Simonsen, H. B. (2020). Assessing Animal Welfare: Where Does Science End and Philosophy Begin? Animal Welfare, 1(4), 257–267, available: doi: 10.7120/09627286.1.3.257.
Schroeder, P., Jones, S., Young, I. S. and Sneddon, L. U. (2014). What do zebrafish want? Impact of social grouping dominance and gender on preference for enrichment. Laboratory Animals, SAGE Publications Ltd, 48(4), 328–337, available: doi: 10.1177/0023677214538239.
Spence, R., Ashton, R. and Smith, C. (2007) Oviposition decisions are mediated by spawning site quality in wild and domesticated zebrafish, Danio rerio. Behaviour, 144(8), 953–966, available: doi: 10.1163/156853907781492726.
Spence, R., Gerlach, G., Lawrence, C. and Smith, C. (2008). The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews, 83(1), 13–34, available: doi: 10.1111/j.1469-185X.2007.00030.x.
Stewart, A., Gaikwad, S., Kyzar, E., Green, J., Roth, A. and Kalueff, A. V. (2012) Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology, 62(1), 135–143, available: doi: 10.1016/j.neuropharm.2011.07.037.
United Kingdom, Department for Environment, Food & Rural Affairs (2013) Guidance/Animal Welfare, available: https://www.gov.uk/guidance/animal-welfare#legislation [accessed 02 May 2019].
Westerfield, M. (2000). The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), 4th ed., Univ. of Oregon Press: Eugene.
White, L. J., Thomson, J. S., Pounder, K. C., Coleman, R. C. and Sneddon, L. U. (2017). The impact of social context on behaviour and the recovery from welfare challenges in zebrafish, Danio rerio. Animal Behaviour, 132, 189–199, available: doi: 10.1016/j.anbehav.2017.08.017.
Woodward, M. A., Winder, L. A. and Watt, P. J. (2019). Enrichment increases aggression in zebrafish. Fishes, MDPI AG, 4(1), available: doi: 10.3390/fishes4010022.
Zabel, C. and Klose, J. (2008) Animal models in human disease. Proteomics - Clinical Applications, 2(5), 635–637, available: doi: 10.1002/prca.200890015.
ZFIN. (2004). ZFIN: Search, available: https://zfin.org/ZDB-GENE-041010-57 [accessed: 18 September 2019].
APPENDIX