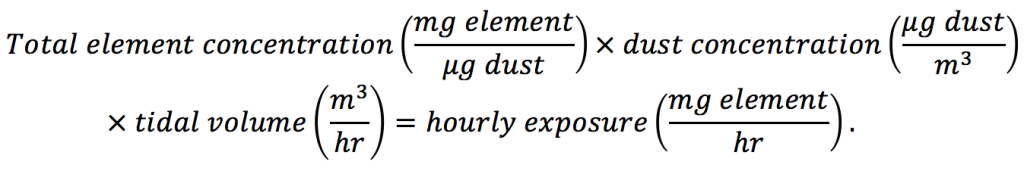Authors: Dallon C. Knight, Nicole A. Ramos, Chris R. Iceman, & Sarah M. Hayes
Date: June 2017
doi:10.22186/jyi.33.1.8-18
Abstract
Recent studies highlight the health risks associated with toxic metal(loid)s [e.g., arsenic (As), zinc (Zn), and lead (Pb)] in dust from mining operations, urban settings, and rural roads. To have a deleterious health effect, inhaled or ingested metal(loid)s must dissolve under conditions in the lung or gastrointestinal tract. In this study, we determined total and physiologically-soluble fractions of metal(loid)s in road dust from four sites in east-central interior Alaska. Total As and antimony (Sb) were enriched up to 26.2 and 53.7, respectively, in dusts relative to average crustal abundance. Several elements such as nickel (Ni), As, and Sb were highly to moderately soluble in simulated lung fluids (7-80%, 15-51%, and 5-42%, respectively). Nickel and As exceeded the EPA inhalation risk unit, which is an exposure level of minimal risk. Despite several elements being highly soluble in simulated gastric fluids, including Ni, copper (Cu), As, and Pb, only As samples exceeded the oral reference dose for children (based on total elemental concentrations) in some samples. The highest exposure risks identified in this study are inhalation of As and Ni present in road dust and ingestion of As-containing dust, especially by children. Additional studies would be needed to further quantify the health risk posed by road dust in this region.
Introduction
Road Dusts
Numerous studies report enrichment of potentially toxic metal(loid)s (e.g., As, cadmium (Cd), Cu, Pb, Zn, and Ni) along roadways of all kinds (e.g., Apeagyei, Bank, & Spengler, 2011; Meza-Figueroa et al., 2016; Witt, Shi, Wronkiewicz, & Pavlowsky, 2014). However, few have focused on dust in (sub)arctic environmental conditions (Brumbaugh, Morman, & May, 2011; Hasselbach et al., 2005; Moghadas et al., 2015; Norman et al., 2016; Shotyk et al., 2016; Walker & Everett, 1987). Road dust is a potential source of human exposure to toxic metal(loid)s (Colombo, Monhemius, & Plant, 2008; DeWitt et al., 2016; Garcia-Rico et al., 2016; Witt et al., 2014) and is of particular concern because the small particles (< 70 µm) are wind-transportable (Gillette & Walker, 1977; Kok, Parteli, Michaels, & Bou Karam, 2012). Small particles (< 45 µm) are commonly also enriched in toxic metal(loid)s (Meunier, Koch, & Reimer, 2011).
Metal(loid) enrichments in road dust have been correlated with a variety of natural and anthropogenic sources (Charlesworth, De Miguel, & Ordonez, 2011). Tires are a source of Zn, and brake parts have high concentrations of iron (Fe) and Cu (Apeagyei et al., 2011). Catalytic converters on vehicles are a source of platinum group elements (Colombo et al., 2008). Use of studded tires, a common practice across Alaska, is also a source of metal(loid)s in road dust (Norman et al., 2016). Studies of metal deposition in paved roadside snow banks in high latitude environments, where snows persist for several months, reported enrichments in Pb, Zn, Cd, Cu, and Ni (Moghadas et al., 2015).
The Alaskan Department of Environmental Conservation has examined road dust in several villages and measured 24-hour averages of particulate matter up to 608 µg m-3, which exceed the federal standard of 150 µg m-3 (AK DEC, 2011). A study that looked at total and bioaccessible metal concentrations along mining haul road in Alaska found enrichment of Zn, barium (Ba), Cd, and Pb in road dust, moss, and other higher vegetation (Brumbaugh et al., 2011), with a spatial extent of at least 25 km in the downwind direction. Another study reported deposition rates of 500 g m-2 (at 8 m from the road), resulting in a layer of dust up to 10 cm deep and measurable deposition of dust at 1000 m from the road along the Dalton Highway, an unpaved road from Fairbanks to Prudhoe Bay, Alaska (Walker, & Everett, 1987). Dust generated on the haul road contains elevated metal(loid) concentrations and has also been linked to local vegetation changes, increased pH near the road, and thawing of ground ice (Myers-Smith, Arnesen, Thompson, & Chapin, 2006; Walker & Everett, 1987). These studies highlight the potential for unhealthy dust concentrations and enrichment of toxic metal(loid)s in road dust around Fairbanks.
Fairbanks has a long history of mining and is located in the Tintina Gold Province, containing a series of epizonal mercury (Hg)-Sb-As-gold (Au) gold vein deposits (Gough & Day, 2007). In addition to the mineralization, mafic and ultramafic lithologies present in the region may be sources of Ni, chromium (Cr), Fe, manganese (Mn), and Co (Gough & Day, 2007; Wang et al., 2007). Wang et al. (2007) also found the surficial soil concentrations of As and Sb ranging between 3 to 410 mg kg-1 and 0.4 to 24 mg kg-1, respectively, elsewhere in Alaska’s interior. In interior Alaska, unpaved roads may sometimes be constructed using mine overburden and waste rock, which could contain elevated concentrations of potentially toxic metal(loid)s (FHA, 2016; H. Schaefer, personal interview, November 16, 2016). Road construction and driving on unpaved roads where potentially toxic elements are present may loft metal(loid)-bearing particles, thereby making them available for ingestion or inhalation.
Health Effects of Toxic Metals
Nutrient and micronutrient metal(loid)s have biochemical and physiological roles within the body, but may also be toxic depending on concentration. However, As, Ni, Sb, and Pb have no known biological function and have well-documented deleterious health effects (e.g., Chang, Magos, & Suzuki, 1996). Exposures are classified as either acute, meaning a short-term, generally higher dose exposure, or chronic, meaning a lower dose that is encountered over a longer period. Both acute and chronic exposures can have deleterious health effects, but chronic exposures are commonly harder to connect directly with health effects.
Arsenic has been reported to disrupt biological processes, including cellular respiration, DNA replication and DNA repair (Tchounwou, Yedjou, Patlolla, & Sutton, 2012). Additionally, As is highly carcinogenic and has been linked to respiratory disease, cardiovascular disease, anemia, gastrointestinal distress, nervous system disorders, and other negative health conditions (ATSDR, 2007). Nickel is also carcinogenic and has been linked to respiratory and renal necrosis, birth defects, immune system alteration, and other disorders (ATSDR, 2005). Antimony is also potentially carcinogenic and has been linked to decreased respiratory function, gastrointestinal distress, optic disorders and reproductive disruption (ATSDR, 1992). These are just a few of the potential health impacts of exposure to three specific metal(loid)s, which lead to concerns regarding elevated levels of potentially toxic metal(loid)s found along roadways.
Although regulation is determined by total elemental concentrations, negative health impacts are controlled by the fraction of metal(loid) solubilized under physiologically relevant conditions or bioaccessibility. Bioaccessibility is controlled by mineralogy, speciation, oxidation state, particle size, and encapsulation (Plumlee, Ziegler, & Lollar, 2005). Small particles (< 45 µm) are typically more bioaccessible than larger particle sizes, likely due to higher surface area (Meunier et al., 2011; Ruby et al., 1999). The two major exposure routes for dust and soil are ingestion or inhalation (Fig. 1). Accidental ingestion may result from hand-to-mouth transfer (especially by children) or food-bound particles (Taylor & Williams, 1995). Particles less than 250 µm typically stick to children’s hands and can be swallowed (EPA, 2012). Inhalation depends largely on particle size. Particles that are less than 4 µm will enter the lungs, and particles that are approximately 2 µm can reach and remain in the alveoli for months to years (Lundborg, Falk, Johansson, Kreyling, & Camner, 1992; Plumlee et al., 2005). Physiologically based extraction tests (PBETs) provide insight to the bioaccessible fraction of metal(loid)s by interaction with gastric or lung fluids (EPA, 2012). PBET analysis is becoming a widely used and verified alternative for animal models in examining bioaccessibility of several common contaminant metal(loid)s (Wragg et al., 2011).
Figure 1. Exposure mechanisms for dust particles.
Goals of This Study
The combination of enrichment of toxic elements in road dust, high numbers of unpaved roads in Alaska, and high concentrations of dust measured on unpaved roads highlights the need for direct examination of road dust to assess the health impact of dust from unpaved roads near growing population centers. This study examines the total and bioaccessible fractions of potentially toxic metal(loid)s in Alaskan road dust collected in interior Alaska, mostly near Fairbanks (Fig. 2). The goal is to compare the total and bioaccessible metal(loid) concentrations to relevant soil screening levels and the EPA reference dose or inhalation unit risk to determine if these road dusts represent a health risk to the Fairbanks population. This study will provide initial assessment of the health risk from unpaved road dust in interior Alaska.
Figure 2. Major Alaskan roads outlined in blue with study areas in red. Expanded views of Fairbanks sample sites and Denali Highway. Images from Google earth and Alaska Department of Transportation (AK DOT, 2016).
Methods
All glassware was acid washed prior to use, and all chemicals were ACS grade or better.
Sampling Sites and Collection Procedures
Dust samples were collected from interior Alaska in summer 2014 using a variety of methods, including passive samplers mounted on the roadside and mounted on a vehicle as well as artificial agitation (Table 1). Sample sites were selected to represent a variety of environments, including: within Fairbanks, AK city limits, residential communities near Fairbanks, and along the Denali Highway, an unpaved secondary highway often used for recreational activities (Fig. 2). Passive samplers (TE-200-PAS from Tisch, Village de Cleves, OH) were continuously deployed for 28 days from July until August. Because of the low dust volumes collected in the passive samples, additional samples were collected at the end of the summer using a leaf blower to agitate dust. The leaf blower was used to loft particles into a clean plastic garbage bag. Low dust accumulations in the passive samples are attributed to the unusually wet conditions during the summer of 2014. From June and the end of August 2014, interior Alaska received 29.5 cm of precipitation. The 30-year average for the same period is 13.7 cm (Wendler, 1995). The Denali Highway sample was collected in a passive sampler mounted on the back of a vehicle during a single round trip from Fairbanks across the Denali Highway from the Cantwell side. Another sample was collected from undisturbed boards underneath a house located approximately 35 m from the proximal road, which is assumed to represent years of metal(loid) deposition.
Size Fractionation
Table 1. Sample descriptions and summary of collection methods.
A brass sieve set (numbers: 10, 18, 35, 60, 120, 200, 325, and 400 mesh, Cole-Parmer USA Standard Test Sieve, Vernon Hills, IN) was used to separate dust particles into size fractions of greater than 2 mm, 2 – 1 mm, 1 – 0.5 mm, 500 – 250 µm, 250 – 125 µm, 125 – 75 µm, 75 – 45 µm, 45 – 37 µm, and smaller than 37 µm. After manual dry sieving each sample to less than 0.5 mm, the remaining sample was added in the top of the stacked sieve set, and the whole assembly was dry-agitated by a sieve shaker (Cole-Parmer SS-3CP, Vernon Hills, IN) on setting 10 for 30 minutes. After 30 minutes, the sieve assembly was removed from the agitator, and each size fraction was weighed, transferred to labeled glass vials, and stored at room temperature. PBETs, described in section 2.4, were performed on the less than 250 µm fraction for gastric and the less than 37 µm fraction for lung extractions.
Total Metal(loid) Concentrations
The total metal(loid) concentrations were determined by dissolving duplicate samples using sodium peroxide sinter followed by inductively-coupled mass spectrometry (ICP-MS) elemental analysis (Cotta & Enzweiler, 2012; Longerich, Jenner, Fryer, & Jackson, 1990; Meisel, Schoner, Paliulionyte, & Kahr, 2002). Briefly, each sample was ground by hand using an agate mortar and pestle until the sample passed through a 200-mesh sieve. To a clean, glassy carbon crucible, 100 mg of sample and 600 mg of sodium peroxide (Alfa Aesar, L11306) were added. This was then mixed thoroughly with a plastic spatula, and an additional 10 – 30 mg of sodium peroxide was sprinkled over the top of the mixture. The crucibles were heated at 480°C for 30 minutes in a muffle furnace, cooled to room temperature, and placed in acid washed Nalgene bottles. To each sample, 10 mL of 18 MΩ H2O was slowly added. Then, 2 mL of 13% HNO3 followed by an additional 2 mL of 35% HCl were added. Additional 18 MΩ H2O was added to bring the total mass of sample, sodium peroxide, acids, and water to 100 g. The samples were diluted by a factor of 10 and analyzed using ICP-MS.
Physiologically-Based Extraction Tests (PBETs)
PBETs have been applied to a variety of samples, including mine wastes (Ruby, Davis, Schoof, Eberle, & Sellstone, 1996; Schaider, Senn, Brabander, McCarthy, & Shine, 2007), reference minerals (Colombo et al., 2008; Lundborg et al., 1992; Takaya et al., 2006), soils (Drysdale et al., 2012), and road dust (Brumbaugh et al., 2011; Dodd, Rasmussen, & Chenier, 2013; Witt et al., 2014). PBETs were performed to determine the fraction of the dust that would dissolve in simulated gastric (EPA, 2012) and alveolar fluids (Drysdale et al., 2012; Takaya et al., 2006). Briefly, a 0.4 M glycine solution adjusted to pH 1.5 using OmniTrace HCl and a freshly prepared modified Gamble’s solution (Drysdale et al., 2012) were heated to 37°C using an incubator-shaker table (Lab-Line 4628; Melrose Park, IL) to mimic the gastric and alveolar fluids, respectively. In a 15 mL Falcon tube, 0.1 g of sieved dust (less than 250 µm for gastric and less than 37 µm for lung) was combined with 10 g of the simulated gastric or lung fluids. These tubes were incubated and shaken at 37°C at 60 rpm in the dark for one hour for the gastric and seven days for the lung extraction. The experiments were terminated by centrifuging at 8,500 g for ten minutes prior to decanting and filtering the supernatant using an acid washed 0.2 µm polypropylene filter (Acrodisc GHP). The pH of the supernatant was measured, and the supernatant was acidified to a pH less than two with OmniTrace nitric acid prior to dilution and analysis by ICP-MS.
Elemental Analysis
Elemental analyses of PBETs and sodium peroxide sinter digestions were performed by ICP-MS. Analyses were performed using a 7500ce ICP-MS (Agilent; Santa Clara, CA) at the Advanced Instrumentation Laboratory (AIL) at the University of Alaska Fairbanks. Both external and internal standards (i.e., scandium (Sc), germanium (Ge), yttrium (Y), rhodium (Rh), and iridium (Ir)) were used in calibration to measure the elements of interest (vanadium (V), Cr, Mn, Ni, Cu, Zn, As, molybdenum (Mo), silver (Ag), Sb, Ba, Pb, and thorium (Th)). Reagent blanks, method blanks, and aqueous standard reference materials (NIST 1640 and SLRS-5) were measured once during the ICP-MS run, in addition to 2% nitric acid blanks and mid-level standards analyzed at least every 15 samples as quality control measures.
Enrichment Factors
Similar to previous studies (e.g., Meza-Figueroa et al., 2016), enrichment factors were calculated based on the average crustal abundance for the given element (Fig. 3; Rundick, 2006). A value above one indicates enrichment, a value of one indicates no enrichment or depletion, and a value below one indicates depletion relative to the average crustal abundance. Total crustal abundance values, shown in Table 2, were used to calculate enrichment factors using the following equation:
(1)
Figure 3. Enrichment factors of dust relative to average crustal abundance. Values above one are enriched whereas values below one are depleted relative to average crustal abundance values tabulated in Table 2.
Health-Based Screening Levels
The Environmental Protection Agency (EPA) of the United States publishes tables of health-based generic screening levels for chemicals, including metal(loid)s of health concern, for the purpose of establishing screening levels at contaminated sites (EPA, 2016). Within these tables, values for oral reference doses (RD) and inhalation reference concentrations (RfCi) can be found, which represent estimates of the maximum daily oral or inhalation dose of a chemical that is “likely to be without an appreciable [noncancerous] risk of deleterious effects during a lifetime,” even for sensitive subgroups (EPA, 2016). Similarly, values are also tabulated for inhalation risks as inhalation unit risk (IUR), representing “the upper bound excess lifetime cancer risk from continuous exposure to an agent at a concentration of 1 µg m-3 in air.” These values will be used to contextualize total elemental concentrations of potentially toxic elements.
Table 2. Total metal(loid) concentrations. For comparison, average crustal abundance, U. S. Environmental Protection Agency residential soil screening levels, oral references doses, and reference inhalation concentrations are also tabulated. Average and one standard deviation of duplicate measurements are reported for unsieved samples. Values below detection limit are indicated by BDL, and values not reported are indicated by NR. a Values for upper continental crust from (Rudnick, 2006). b From Regional Screening Level (RSL) Resident Soils Table (EPA, 2016). c Tabulated as reference dose (RfD0) or SFO, an estimate of a daily oral exposure to the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime in EPA (2016). d Tabulated as inhalation unit risk (IUR), an upper bound excess lifetime cancer risk estimated from continuous exposure to an agent at a concentration of 1 µg m-3 in air (EPA, 2016). e Tabulated as chronic inhalation reference concentration (RfCi; EPA, 2016).
Comparison of Data with Ingestion Reference Dose
In order to compare measured values with oral reference dose (Table 2), several assumptions are required to arrive at the same units. These calculations were performed using a precautionary approach and are intended to provide an upper safe exposure limit. However, the calculations presented do not account for any hand-to-mouth transfer of metal(loid)-bearing particles or enrichment of metal(loid)s in smaller size fractions that are more readily lofted by vehicles. For ingestion calculations, it was assumed that all inhaled dust particles were captured in the mouth, nose and throat, and ingested rather than inhaled. The data shown in Figure 4A estimates the mass of each metal(loid) in air an adult may be exposed to per hour of metal(loid) exposure [Eq. (2)]:
(2)
where total metal(loid) concentrations from Table 2 were converted to milligram metal(loid) per microgram dust. The dust concentration, mass of dust per volume air, was assumed to be 300 µg m-3, a value arrived at using roughly half the highest measured dust concentration in an Alaskan village (608 µg m-3) and double the state and federal exposure standard (24-hour average of 150 µg m-3; AK DEC, 2011). Ingestion exposure calculations [Eq. (2)] require an estimation of the air volume exchange by an average adult (80.7 kg and 15 breaths min-1) or child (20 kg and 20 breaths min-1) assuming that 7 mL kg-1 of air is exchanged (Fleming et al., 2011; McDowell, Fryar, Ogden, & Flegal, 2008; Ricard, 2003). This calculation yielded 0.508 m3 hr-1 and 0.168 m3 hr-1 tidal volume for adults and children, respectively. The individual element hourly exposures were calculated using adult tidal volumes.
The daily exposure reference dose (listed in Table 2) were converted to milligram metal(loid) per hour and adjusted for body mass for comparison with measured values using [Eq. (3)] and shown in Figure 4A:
(3)
Comparison of Data with Inhalation Unit Risk
Dust exposure from inhalation (Fig. 4B) was calculated by multiplying the concentration of metals in dust by the concentration of dust, according to the following equation, and directly compared with inhalation unit risk listed in Table 2:
(4)
This assumes that all material was of a particle size suitable for inhalation and was all inhaled into the lungs and remained in residence for seven days, and that none of the inhaled material was trapped in the upper respiratory tract and ingested.
Percent Bioaccessibility
The percent bioaccessibility (Table 3) was calculated to better evaluate the fraction of the total element present in the sample liberated in simulated physiological conditions. Reported errors were propagated using the standard deviations of replicate measurements of the total and bioaccessible metal(loid)s using standard methods (Harris, 2010). The following equation was used to calculate percent bioaccessibility:
(5)
Results
Total Metal Concentrations
Figure 4. Health risk associated with road dust. Exposure potentials for each sample in addition to EPA reference doses or concentrations are shown for A. gastric, and B. inhalation exposures to road dust.
The dust examined were generally below the EPA residential soil screening levels, based on the total elemental analysis results shown in Table 2 (EPA, 2015). However, all of the dust exceed the screening levels for As and possibly Cr. The reference dose for Cr depends on the oxidation state of Cr, and Cr (III) has a much higher screening level than Cr (IV) as shown in Table 2. Most Cr-bearing minerals contain Cr (III) and would, thus, not exceed screening levels. Additionally, several soils were above the screening level for V, Mn, As and Sb. There are also distinct differences in the elemental content of the two Gold Hill Rd. dust samples, with the dust from under the house being higher in all elements except Sb, Mo, and As (Table 2).
Compared with the average crustal abundance (Table 2), which results in enrichment factors shown in Figure 3, all samples are at least somewhat enriched in As, Ag, Mo, and Sb, and most are enriched in Mo and Ba. The highest enrichment factors were observed in As and Sb, the two elements with the lowest oral reference doses (Table 2), which is indicative of their toxicity. However, these values are within the range of As and Sb values for surficial soils previously reported elsewhere in the Tintina Gold Province (Wang et al., 2007). The highest observed enrichment factors were for As and Sb in rural residential road dust (Moose Mountain and Gold Hill Rd.; Fig. 3). The Denali Highway sample is enriched relative to crustal abundance in Cu, Zn, As, Mo, Ag, Sb, Ba, Pb, and Th. In contrast, only As, Mo, Ag, Sb, and Ba are enriched relative to crustal abundance in the O’Connor Rd (Fig. 3). Of the two Gold Hill Rd. samples, the dust sample from under the house is closer to average crustal abundances for nearly all elements than the road dust sample (Fig. 3).
When total toxic metal(loid) composition is compared with EPA reference doses or concentrations, the potential gastric exposures are well below the reference doses for all elements except As, for which total concentrations exceed the reference dose for children at the Moose Mountain Rd. and Gold Hill Rd. sites (Fig. 4A). However, for inhalation, road dust also exceed the inhalation risk unit for Ni and for As in some samples (Fig. 4B).
Bioaccessibility
Gastric bioaccessibility, shown in Table 3, demonstrates that the elements examined have a wide range of bioaccessibility. Vanadium, Cr, Mo, and Th have low gastric bioaccessibility with less than 4% of the total element solubilized in simulated gastric fluids. Other elements, including Ba, Zn, and Sb exhibit low to medium bioaccessibility with 1-22% gastric liberation. Moderate to high gastric bioaccessibility was observed in Mn, As, and Ag (5-60% liberation). Consistently high bioaccessibility was observed in Ni, Cu, and Pb with gastric liberations up to 82%. The percent bioaccessibility of metal(loid)s in artificial lung fluid extractions are also shown in Table 3, and in general, the lung bioaccessibility is much lower than in the gastric extractions. Elements with concentrations below detection limits for all samples (Cr, Mn, Zn, Ag, Pb, and Th) were excluded from the table. Several other elements had lung bioaccessibility < 5% for all samples, including V, Cu, and Ba, with most values less than 1%, indicating low bioaccessibility. Molybdenum lung bioaccessibility was mostly below detection limits, but 21% of Mo was liberated in the Denali highway sample. This sample has a similar total Mo concentration as other samples, thus the differences in bioaccessibility points to mineralogical control over physiological solubility. Moderate to high bioaccessibility were observed for Ni, As, and Sb at 7-80%, 15-52%, and 5-43% bioaccessibility, respectively. These trends are not consistent with the trends observed in the gastric extractions.
Table 3. Percent gastric and lung bioaccessibility of metal(loid)s. Values are reported as the average percent solubility relative to the total and one standard deviation of triplicate measurements. Physiologically-based extraction tests were performed on less than 250 µm for gastric and less than 37 µm for lung extractions isolated by dry sieving. For lung bioaccessibility, Cr, Mn, Zn, Ag, Pb, and Th were excluded from the table because all extracted solutions were below detection limits.
Discussion
Given the mafic and ultra-mafic lithologies and mineralization in the Fairbanks region, it is perhaps unsurprising that As and Sb are enriched in all samples (Fig. 3). The urban residential site of the O’Connor Road is the least contaminated with only moderate enrichments (Fig. 3). This sample site has higher population density, and thus, the dust has the largest potential to affect residents. It is positive that it was found to be the less enriched in As, Sb, and other potentially toxic elements. The elemental profile of the Gold Hill Under House site dust is much more similar to average crustal abundances than other samples, including Gold Hill Road dust, potentially indicating multiple sources of dust contribute to the dust accumulation under the house.
Conversely, the Denali Highway site has the highest number of elements enriched, although enrichments were modest. Despite the isolated location, this unpaved highway receives heavy use during the summer, especially during hunting seasons by off road vehicles. Since many of the vehicles traveling the road are not equipped with microparticulate air filtration, these travelers could be exposed to a substantial amount of dust that is enriched in toxic elements (Fig. 1) at concentrations that are near or above EPA reference doses (Fig. 4). All-terrain vehicles have been observed to loft up to 160 mg m-3 of less than 10 µm particles at the breathing level of ATV operators (Goossens & Buck, 2014). The same study demonstrated that these particles remain suspended for at least one minute, creating excellent conditions for inhalation and ingestion of suspended particulates. Thus, land use is an important factor when assessing acute and chronic exposures. The Denali Highway site would likely provide acute exposure while recreating whereas residents near the Moose Mountain Rd., Gold Hill Rd., or O’Conner Rd. sites would more likely be exposed to chronic doses.
The total and bioaccessible fractions of toxic metal(loid)s in Alaskan road dust were collected to assess if these road dust represent a health threat to nearby communities. Schaider et al. (2007) observed generally higher rates for gastric rather than lung bioaccessibility in Pb and Zn reference minerals, which is consistent with the findings of this study.The wide range of metal(loid)s liberated under physiological conditions is typical because bioaccessibility depends on particle size, identity of metal(loid)-bearing mineral phases, metal(loid) speciation, exposure of metal(loid)s to extraction fluid, and identity of extraction fluid (Ruby et al., 1999). Evidence of this point is seen in the very different rates of metal(loid) release that are observed in the fraction of metal(loid)s liberated in the PBET literature. In addition, the variety of PBET procedures may also affect the results, as has been reported in a review of 96 lung bioaccessibility articles (Wiseman, 2015).
Health Risk Associated With Alaskan Road Dust
This study identifies Ni and As as possible inhalation health risks and As as a potential ingestion risk based on the potential for exceeding recommended reference doses or concentrations (Fig. 4). Several elements were quite soluble under simulated gastric conditions, indicating they could be bioaccessible, most notably Ni, Cu, and Pb (Table 3). Nickel and Cu are micronutrients; however, Pb has no known biological function and is toxic and highly soluble under gastric conditions. This highlights that bioaccessibility largely controls the potential for toxicity and should be considered.
Arsenic and Sb show enrichment in all samples (Fig. 3), especially the rural residential samples, and both are highly toxic metalloids with low inhalation concentrations and the lowest oral reference doses of all elements examined in this study (Table 2). Both Sb and As form trivalent and pentavalent oxyanions in the surficial environment. In both cases, the trivalent form is both more mobile and more toxic than the pentavalent form, and more reduced forms are even less soluble and toxic (ASTDR, 1992; Ruby et al., 1999). The connection between As mineralogy and bioaccessibility has been extensively examined. Arsenic bioaccessibility can be summarized as: arsenopyrite is less bioaccessible than As-bearing Fe (oxy)hydroxides and Mn oxides, which is less bioaccessible than As oxides and oxyanions (Ruby et al., 1999). Substantially less work has been performed on Sb bioaccessibility, but Sb in 16 soil types yielded low (< 10%) gastric bioavailability in juvenile swine models (Denys et al., 2012). Additionally, a low correlation was observed between the bioaccessibility in swine models and simulated gastric extractions, which liberated up to 18% Sb. Other studies have reported up to 6% liberation of Sb from mine tailings in simulated gastric extractions (Li et al., 2014). The authors are not aware of any studies examining liberation of Sb in lung fluids.
In simulated lung fluids, Drysdale et. al (2012) reported Ni bioaccessibility of less than 4.2% in soils from a mining and smelting-impacted region. Others have examined Ni and Ni oxide solubility in artificial lysosomal lung fluids and found up to 88% release of NiO within 24 hrs (Mazinanian, Hedberg, & Wallinder, 2013). Again, the range of these results highlights the importance of speciation.
In this study, moderate to high rates of Pb liberation from gastric solutions were observed (16-82%), whereas no liberation was detected in simulated lung fluids. Schaider et al. (2007) observed widely variable rates of gastric liberation in reference minerals PbS and PbCO3 (3% and 97%, respectively). The same study reports lung bioaccessibilities of 0.4 and 14%, for PbS and PbCO3, respectively, using a PBET solution mimicking conditions inside alveolar macrophages. The high bioaccessibility lends insight into the Pb mineralogy, as many studies have examined the link between Pb mineralogy and bioaccessibility or bioavailability (Casteel, Weis, Henningsen, & Brattin, 2006; Ruby et al., 1996; Ruby et al., 1999). The most bioaccessible phases with gastric liberation values near 100% include lead associated with Mn oxides and PbCO3, and medium bioaccessibility (~50%) include lead associated with Fe (oxy)hydroxides and Pb phosphates (Casteel et al., 2006), which may be present in these samples. Although high bioaccessibility is somewhat concerning due to the potential for hand-to-mouth transmission and ingestion of Pb-bearing particles, the total Pb concentrations in these dust are well below the EPA soil screening level for residential soils (Table 2).
Limitations of Study
The limited number of samples and variety of sampling methods employed were the principal limitations of this study. While a variety of sampling methods were necessary to collect enough sample mass, this may affect the apparent composition of dust samples. Ideally, dust samples would all have been collected passively. The unusually wet weather in summer 2014 may have lowered the number of airborne particulate concentrations and may have also facilitated surface water transport of small particles that would otherwise have been available for lofting. Additionally, the moisture present may have altered the mineralogical composition of dust or rainwater may flush ions that might otherwise form small, bioaccessible, readily lofted mineral salts at the road surface. Artificial agitation was capable of lofting larger particles than a passing vehicle. Thus, sieving was used to minimize the impact of this on the study results, and the results presented here are expected to be illustrative of potential human exposures.
This work relied on the ability of PBETs to predict the bioaccessibility of a variety of elements. Whereas PBETs are an attractive method for supplementing resource-intensive animal model studies, they have not been validated for all elements. PBETs have been extensively applied to assessing the bioaccessibility of Pb, As, and Ni in geological media, but less work has been done on Sb (Denys et al., 2012; Ng, Juhasz, Smith, & Naidu, 2013; Wiseman, 2015; Wragg et al., 2011). Despite the method not having validation for all elements, the method still lends qualitative insight into solubility of elements under physiological conditions for elements that have yet to be validated.
Conclusions
This work provides an initial assessment of the health risk represented by metal(loid)s in Alaskan road dust. The dust was consistently enriched in As, Ag, and Sb, but perhaps the most significant risk observed based on comparisons to reference doses or concentrations is from inhalation of As and Ni or ingestion of As, especially at the rural residential locations where As is most enriched. These results are consistent with the medium to high bioaccessibility of As and Sb in lung fluids and As in the gastric extraction. Lead is highly bioaccessible in the gastric extraction but is not identified as a health risk based on total Pb concentrations. Although road dust is not the only source of dust to residents, this study shows that road dust can be highly enriched in As and Sb, and highlights the potential for health risks associated with chronic exposure to road dust from the Fairbanks area.
Additional measurements should be conducted using passive samplers to collect dust dispersed by passing vehicles rather than artificial agitation, which were used in this study due to the wet summer. Additionally, a direct measurement of lofted particulate matter concentrations at adult and child breathing levels would further refine the assumptions used in this work. It is clear that further study of exposure risks is warranted to evaluate the risk posed to residents.
Acknowledgements
The authors gratefully acknowledge the contributions of Karen Spaleta at the Advanced Instrumentation Laboratory (AIL) at the University of Alaska Fairbanks for excellent technical support. Material support for this work was provided by the University of Alaska Fairbanks Office of Undergraduate Research & Scholarly Activity (URSA) Student Project Award and Biomedical Learning and Student Training (BLaST) Scholars Program. Work reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under three linked awards number RL5GM118990, TL4 GM 118992 and 1UL1GM118991.
References
AK DEC, Alaska Department of Environmental Conservation (2011). A preliminary assessment of fugitive dust from roads in eight Alaskan villages in the Northwest Arctic Borough. Retrieved September 1, 2016 from https://dec.alaska.gov/air/am/projects&Reports/dust_NWAB_03-05.pdf
AK DOT, Alaska Department of Transportation. Route Summary. Retrieved November 19, 2016 from http://511.alaska.gov/alaska511/routeSummary/
Apeagyei, E., Bank, M. S., & Spengler, J. D. (2011). Distribution of heavy metals in road dust along an urban-rural gradient in massachusetts. Atmospheric Environment, 45(13), 2310-2323. doi:10.1016/j.atmosenv.2010.11.015
ASTDR, Agency for Toxic Substances and Disease Registry (1992). Toxicological profile for antimony and compounds. Retrieved August 24, 2016, from http://www.atsdr.cdc.gov/toxprofiles/tp23.pdf
ASTDR, Agency for Toxic Substances and Disease Registry (2007). Toxicological profile for Arsenic. Retrieved November, 2016, from http://www.atsdr.cdc.gov/ToxProfiles/tp2.pdf
ASTDR, Agency for Toxic Substances and Disease Registry (2005). Toxicological profile for Nickel. Retrieved November, 2016, from http://www.atsdr.cdc.gov/ToxProfiles/tp15.pdf
Brumbaugh, W. G., Morman, S. A., & May, T. W. (2011). Concentrations and bioaccessibility of metals in vegetation and dust near a mining haul road, Cape Krusenstern National Monument, Alaska. Environmental Monitoring and Assessment, 182(1-4), 325-340. doi:10.1007/s10661-011-1879-z
Casteel, S. W., Weis, C. P., Henningsen, G. M., & Brattin, W. J. (2006). Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environmental Health Perspectives, 114(8), 1162-1171.
Chang, L. W., Magos, L., & Suzuki, T. (1996). Toxicology of metals. Boca Raton, FL: Lewis.
Charlesworth, S., De Miguel, E., & Ordonez, A. (2011). A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environmental Geochemistry and Health, 33(2), 103-123. doi:10.1007/s10653-010- 9325-7
Colombo, C., Monhemius, A. J., & Plant, J. A. (2008). Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicology and Environmental Safety, 71(3), 722-730. doi:10.1016/j.ecoenv.2007.11.011
Cotta, A. J. B., & Enzweiler, J. (2012). Classical and new procedures of whole rock dissolution for trace element determination by ICP-MS. Geostandards and Geoanalytical Research, 36(1), 27-50. doi:10.1111/j.1751-908X.2011.00115.x
Denys, S., Caboche, J., Tack, K., Rychen, G., Wragg, J., Cave, M., Jondreville, C., & Feidt, C. (2012). In vivo validation of the unified BARGE method to assess the bioaccessibility of arsenic, antimony, cadmium, and lead in soils. Environmental Science and Technology, 46(2), 6252-6260. doi:10.1021/es3006942
DeWitt, J., Buck, B., Goossens, D., Hu, Q., Chow, R., David, W., Young, S., Teng, Y. X., Leetham- Spencer, M., Murphy, L., Pollard, J., McLaurin, B., Gerads, R., & Keil, D. (2016). Health effects following subacute exposure to geogenic dusts from arsenic-rich sediment at the Nellis Dunes Recreation Area, Las Vegas, NV. Toxicology and Applied Pharmacology, 304, 79-89. doi:10.1016/j.taap.2016.05.017
Dodd, M., Rasmussen, P. E., & Chenier, M. (2013). Comparison of two in vitro extraction protocols for assessing metals’ bioaccessibility using dust and soil reference materials. Human and Ecological Risk Assessment: An International Journal, 19(4), 1014-1027. doi:10.1080/10807039.2012.719381
Drysdale, M., Bjorklund, K. L., Jamieson, H. E., Weinstein, P., Cook, A., & Watkins, R. T. (2012). Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluid. Environmental Geochemistry and Health, 34(2), 279-288. doi:10.1007/s10653-011-9435-x
EPA, Environmental Protection Agency (2012). Standard operating procedure for an in vitro bioaccessibility assay for lead in soil. Retrieved September 27, 2015, from https://semspub.epa.gov/work/HQ/174533.pdf
EPA, Environmental Protection Agency (2016). Regionals screening levels (RSL) summary table. Retrieved July 6, 2016, from https://www.epa.gov/risk/regional-screening-levels-rsls- generic-tables-may-2016
FHA, Federal Highway Administration (2016). User Guidelines for Waste and Byproduct Materials in Pavement Construction. Retrieved November 12, 2016, from https://www.fhwa.dot.gov/publications/research/infrastructure/pavements/97148/039.cfm
Fleming, S., Thompson, M., Stevens, R., Heneghan, C., Plüddemann, A., Maconochie, I., Tarassenko, L., & Mant, D. (2011). Normal ranges of heart rate and respiratory rate in children from birth to 18 years: a systematic review of observational studies. Lancet, 377(9770), 1011–1018. http://doi.org/10.1016/S0140-6736(10)62226-X
Garcia-Rico, L., Meza-Figueroa, D., Gandolfi, A. J., Del Rio-Salas, R., Romero, F. M., & Meza- Montenegro, M. M. (2016). Dust-metal sources in an urbanized arid zone: Implications for health-risk assessments. Archives of Environmental Contamination and Toxicology, 70(3), 522-533. doi:10.1007/s00244-015-0229-5
Gillette, D. A., & Walker, T. R. (1977). Characteristics of airborne particles produced by wind erosion of sandy soil, high plains of West Texas. Soil Science, 123, 97-110.
Goossens, D. & Buck, B. (2014). Dynamics of dust clouds produced by off-road vehicle driving. Journal of Earth Sciences and Geotechnical Engineering, 4(2), 1-21.
Gough, L. P., & Day, W. C. (2007). Tintina Gold Privince study, Alaska and Yukon Territory, 2002- 2007: Uncerstanding the origin, emplacement, and environmental signature of mineral resources. US Geological Survey Fact Sheet. Retrieved August 10, 2016, from http://pubs.usgs.gov/fs/2007/3061/
Harris, D. C. (2010). Quantitative chemical analysis. New York: W. H. Freeman.
Hasselbach, L., Ver Hoef, J. M., Ford, J., Neitlich, P., Crecelius, E., Berryman, S., Wolk, B., & Bohle, T. (2005). Spatial patterns of cadmium and lead deposition on and adjacent to National Park service lands in the vicinity of Red Dog Mine, Alaska. Science of the Total Environment, 348(1-3), 211-230. doi:10.1016/j.scitotenv.2004.12.084
Kok, J. F., Parteli, J. R., Michaels, T. I., & Bou Karam, D. (2012). The physics of wind-blown sand and dust. Reports on Progress in Physics, 75(106901).
Li, J., Wei, Y., Zhao, L., Zhang, J., Shangguan, Y., Li, F., & Hou, H. (2014). Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicology Environmental Safety, 110, 308-315. doi: 10.1016/j.ecoenv.2014.09.009.
Longerich, H. P., Jenner, G. A., Fryer, B. J., & Jackson, S. E. (1990). Inductively coupled plasma- mass spectrometric analysis of geological samples: A critical evaluation based on case studies. Chemical Geology, 83(1–2), 105-118. doi: 10.1016/0009-2541(90)90143-U
Lundborg, M., Falk, R., Johansson, A., Kreyling, W., & Camner, P. (1992). Phagolysosomal pH and dissolution of cobalt oxide particles by alveolar macrophages. Environmental Health Perspectives, 97, 153-157.
Mazinanian, N., Hedberg, Y., & Wallinder, I. O. (2013). Nickel release and surface characteristics of fine powders of nickel metal and nickel oxide in media of relevance for inhalation and dermal contact. Regulatory Toxicoliogy and Pharmacology, 65 (1), 135-146.doi: 10.1016/j.yrtph.2012.10.014
McDowell, M. A., Fryar, C. D., Ogden, C. L., & Flegal, K. M. (2008). Anthropogenic reference data for children and adults: United States, 2003-2006. National Health Statistics Reports, 10, 1-48.
Meisel, T., Schoner, N., Paliulionyte, V., & Kahr, E. (2002). Determination of rare earth elements, Y, Th, Zr, Hf, Nb, and Ta in geological reference materials G-2, G-3, SCO-1 and WGB-1 by sodium peroxide sintering and inductively coupled plasma mass spectrometry. Geostandards and Geoanalytical Research, 26(1), 53-61. doi:10.1111/j.1751- 908X.2002.tb00623.x
Meunier, L., Koch, I., & Reimer, K. J. (2011). Effect of particle size on arsenic bioaccessibility in gold mine tailings of Nova Scotia. Science of the Total Environment, 409(11), 2233-2243. doi:10.1016/j.scitotenv.2011.02.006
Meza-Figueroa, D., Gonzalez-Grijalva, B., Del Rio-Salas, R., Coimbra, R., Ochoa-Landin, L., & Moreno-Rodriguez, V. (2016). Traffic signatures in suspended dust at pedestrian levels in semiarid zones: Implications for human exposure. Atmospheric Environment, 138, 4- 14. doi:10.1016/j.atmosenv.2016.05.005
Moghadas, S., Paus, K. H., Muthanna, T. M., Herrmann, I., Marsalek, J., & Viklander, M. (2015). Accumulation of traffic-related trace metals in urban winter-long roadside snowbanks. Water Air and Soil Pollution, 226(12). doi:10.1007/s11270-015-2660-7
Myers-Smith, I. H., Arnesen, B. K., Thompson, R. M., & Chapin, F. S. (2006). Road dust and its environmental impact on Alaskan taiga and tundra. Ecoscience, 13(4), 503-510.
Ng, J. C., Juhasz, A., Smith, E., & Naidu, R. (2013). Assessing the bioavailability and bioaccessibility of metals and metalloids. Environmental Science and Pollution Research Environ Sci Pollut Res, 22(12), 8802-8825. doi:10.1007/s11356-013-1820-9
Norman, M., Sundvor, I., Denby, B. R., Johansson, C., Gustafsson, M., Blomqvist, G., & Janhäll, S. (2016). Modelling road dust emission abatement measures using the NORTRIP model: Vehicle speed and studded tyre reduction. Atmospheric Environment, 134, 96-108. doi:10.1016/j.atmosenv.2016.03.035
Plumlee, G. S., Ziegler, T. L., & Lollar, B. S. (2005). The medical geochemistry of dusts, soils, and other earth materials. In H. D. Holland & K. K. Turekian (Eds.), Environmental Geochemistry (Vol. 9, pp. 263-310). Amsterdam: Elsevier.
Ricard, J. D. (2003) Are We Really Reducing Tidal Volume—And Should We? American Journal of Respiratory and Critical Care Medicine, 167(10), 1297-1298. doi: 10.1164/rccm.2303003
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science & Technology, 30(2), 422-430.
Ruby, M. V., Schoof, R., Brattin, W., Goldade, M., Post, G., Harnois, M., Mosby, D. E., Casteel, S. W., Berti, W., Carpenter, M., Edwards, D., Cragin, D., & Chappell, W. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science & Technology, 33(21), 3697-3705. doi:10.1021/es990479z
Rudnick, R. L., Gao, S. (2006). Composition of the continental crust. In R. L. Rudnick (Ed.), The Crust (2nd ed., Vol. 3, pp. 1-64). New York: Elsevier.
Schaider, L. A., Senn, D. B., Brabander, D. J., McCarthy, K. D., & Shine, J. P. (2007). Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure, and bioavailability. Environmental Science & Technology, 41(11), 4164-4171.
Shotyk, W., Bicalho, B., Cuss, C. W., Duke, M. J. M., Noernberg, T., Pelletier, R., Steinnes, E., & Zaccone, C. (2016). Dust is the dominant source of “heavy metals” to peat moss (Sphagnum fuscum) in the bogs of the athabasca bituminous sands region of Northern Alberta. Environment International, 92-93, 494-506. doi:10.1016/j.envint.2016.03.018
Takaya, M., Shinohara, Y., Serita, F., Onon-Ogasawara, M., Totaki, N., Toya, T., Takata, A., Yoshidda, K., & Kohyama, N. (2006). Dissolution of functional materials and rare earth oxides into pseudo alveolar fluid. Industrial Health, 44, 639-644.
Taylor, D. & Williams, D. (1995). Trace element medicine and chelation therapy. Camrbidge: The Royal Soceity of Chemistry. doi:10.1039/9781847552198-FX001
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy Metal Toxicity and the Environment. In A. Luch (Ed.), Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology (pp. 133-164). Basel: Springer Basel.
Walker, D. J., & Everett, K. (1987). Road dust and its environmental impact on alaskan taiga and tundra. Arctic and Alpine Research, 19(4), 479-489.
Wang, B., Gough, L. P., Wanty, R. B., Crock, J. G., Lee, G. K., Day, W. C., & Vohden, J. (2007). Landscape geochemistry near mineralized areas of eastern Alaska: Chapter H in recent U.S. Geological survey studies in the Tintina gold province, Alaska, United States, and Yukon, Canada--results of a 5-year project (2007-5289H). Retrieved from Reston, VA: http://pubs.er.usgs.gov/publication/sir20075289H
Wendler, G. (1995). Alaska climate reserarch center. Retrieved March 7, 2016, from AKClimate.org
Wiseman, C. L. (2015). Analytical methods for assessing metal bioaccessibility in airborne particulate matter: A scoping review. Analytica Chimica Acta, 877, 9-18. doi:10.1016/j.aca.2015.01.024
Witt, E. C., Shi, H., Wronkiewicz, D. J., & Pavlowsky, R. T. (2014). Phase partitioning and bioaccessibility of Pb in suspended dust from unsurfaced roads in missouri—a potential tool for determining mitigation response. Atmospheric Environment, 88, 90-98. doi:10.1016/j.atmosenv.2014.02.002
Witt, E. C., Wronkiewicz, D. J., Pavlowsky, R. T., & Shi, H. (2013). Trace metals in fugitive dust from unsurfaced roads in the Viburnum Trend resource mining district of Missouri-- implementation of a direct-suspension sampling methodology. Chemosphere, 92(11), 1506-1512. doi:10.1016/j.chemosphere.2013.04.012
Wragg, J., Cave, M., Basta, N., Brandon, E., Casteel, S., Denys, S., Gron, C., Oomen, A., Reimer, K., Tack, K., & Van de Wiele, T. (2011). An inter-laboratory trial of the unified barge bioaccessibility method for arsenic, cadmium and lead in soil. Science of the Total Environment, 409(19), 4016-4030. doi:10.1016/j.scitotenv.2011.05.019