Authors: Brittany M. Thornton, Jessie L. Knowlton, Wendy A. Kuntz
Institution: University of Colorado at Boulder, Michigan Technological University, Kapi' olani Community College, University of Hawai'i
ABSTRACT
Dominance hierarchies have long been observed in nature across many different species. These hierarchies often arise via agonistic behaviors, whereby animals compete for resources while minimizing their physical conflict. Understanding these interactions is important for determining factors that contribute to community structure. We examined interspecific dominance hierarchies of frugivorous neotropical birds at feeding stations baited with bananas in the Wilson Botanical Garden of the Las Cruces Biological Station in Costa Rica. At each feeding station, we used a video camera to record all birds that came to the station, the amount of time each spent on the platform, and all displacement behaviors, including which species was the aggressor and which was displaced. An analysis of 390min of video footage and visual observations revealed a strong linear interspecific dominance hierarchy in the 13 species that came to the feeding stations. This dominance hierarchy largely correlated with species’ body weights, with heavier species being more dominant. More dominant species were also found to spend a greater amount of time on the platforms than subordinate birds. These results suggest that size is one of the most important factors in creating dominance patterns. This can help explain how species with similar diets coexist, as well as predict which species might be most threatened if resources become scarce.
INTRODUCTION
In animal behavior, dominance is defined as the status of a consistent winner of agonistic interactions (Drews, 1993). Differences in dominance levels among individuals are common both within and between species (French & Smith, 2005). These differences in dominance can create a ranking system, forming a social hierarchy in the community (Chase, 1982). Within species, these dominance hierarchies directly influence the distribution of resources, such as food and mates (Hawley, 1999). Between species, these hierarchies are an important factor in determining the size of species’ realized niches and help to shape the structure of entire communities of species (Vandermeer, 1972). For example, interspecific dominance hierarchies can contribute to the maintenance of high species diversity in an ecosystem by allowing individuals to coexist, even if they have overlapping niches (Tilman, 2004). Dominance hierarchies are important not only at the species and community levels, but at the individual level as well. The position of an individual in a dominance hierarchy is hugely important, as it can determine access to resources such as food, mates, shelter and breeding sites (Morse, 1974; Daily & Ehrlich, 1994).
Dominance hierarchies can either be transient, forming at a patchy resource or in times of resource scarcity (Rowell, 1966), or develop early and last over an individual’s lifetime (Chase, 1982). These hierarchies are often formed by interference competition, whereby one individual will use physical aggression or agonistic displays to intimidate a competitor away from a resource (French & Smith, 2005). Agonistic displays are predicted to be used more often than physical conflict because both the dominant and subordinate individuals should benefit from the reduced risk of a physical altercation, in part due to the time and energy saved by not engaging in combat (Bernstein & Ehardt, 1985). Larger individuals are often the winners in interference competition, and thus dominance rank is often correlated with body size or mass in a linear fashion (French & Smith, 2005). Tropical frugivorous birds provide excellent model organisms with which to study dominance hierarchies in species with overlapping niches because this guild of birds is very high in species richness. Many frugivorous bird species have undergone convergent evolution and thus share similar body shapes and sizes (Snow, 1981). This attribute makes them ideal to study how size differences can influence dominance rank.
Costa Rica is home to many tropical fruit specialists as well as birds that function as opportunistic omnivores (Pratt, 1984). In this study, we investigated the influence of species and size on resource accessibility at feeding stations located around the Wilson Botanical Garden at Las Cruces Biological Station in Costa Rica. The Wilson Botanical Garden provided an attractive study site due to the large number of bird species that frequent the area. Twenty years ago, Daily and Ehrlich (1994) studied the influence of social status on foraging behavior and community structure of tropical frugivorous birds in natural versus human-dominated habitats in this same location. They observed birds feeding at fruiting trees during the dry season and found that species with larger bill lengths and body sizes showed greater dominance levels and were able to gain greater access to resources. These more dominant species were also able to forage further from primary forest than subordinate species (Daily & Ehrlich, 1994).
Our study differs from that of Daily and Ehrlich (1994) because we observed an overlapping but unique suite of frugivorous bird species interacting at controlled feeding stations during the wet season. We wanted to compare our dominance hierarchy findings with those of Daily and Ehrlich (1994) to determine if a different suite of species during a different time of year at novel food sources would show the same pattern of larger individuals being more dominant in the feeding hierarchy. We set out to test if even slight differences in body size among frugivores would influence their dominance rank. We hypothesized that even small size differences would yield differences in dominance levels, since these species have overlapping niches and yet are able to coexist.. We speculated that larger species would spend more time on the feeding platforms than smaller species, and larger species would displace smaller species more often, thus creating a linear dominance hierarchy where dominance level was correlated with body size. Most research to date has focused on conspecific dominance hierarchies, however this study is unique because we used a controlled setting to determine how dominance hierarchies are structured within a species-rich community of neotropical frugivorous birds with overlapping niches.
METHODS
Study Area
Our study was conducted at the Las Cruces Biological Station, located in Coto Brus County in southern Costa Rica (8° 47' 7'' N, 82° 57' 32'' W). The station encompasses over 300ha of premontane wet forest habitat ranging in elevation from 1,000 to 1,400m. More than 400 species of birds can be found in this environment, making it a prime study site for ornithologists. By using an established feeding station baited with bananas and other fruit and creating a radius of new feeding stations around it, we were able to observe social dominance in a controlled setting. We placed feeding stations within the Wilson Botanical Garden, a large 16ha floral collection located in the heart of the Las Cruces Biological Station.
Sampling Design
Establishing Feeding Stations
A popular bird feeding station, located near the dining area of the Las Cruces Biological Station within the Wilson Botanical Garden, has been in use for roughly 15 years as a place for local neotropical bird species to perch and eat fruit (Z. Zahawi, personal communication, June 11, 2013). Using this feeding station as our starting point, we plotted circles around it at 25m intervals using ArcMap (Version 9.3.1.; Environmental Systems Resource Institute, 2009). We then placed an additional 12 feeding stations to the east and west of the established station at intervals of roughly 25m, based on logistical constraints (Figure 1). Platforms were placed 1m from trees with low branches to provide escape vegetation. The stations were constructed out of bamboo poles (1 - 1.5m) cut from the invasive bamboo forest in the area. On the top of each bamboo pole we nailed a 25 x 20cm plywood platform and assigned each station an individual name or number. Bananas were placed at each feeding station daily for 1wk before behavioral observations began, to condition birds in the area to come to the new stations. During the initial week of setup we recorded if birds had eaten from the bananas between the hours of 5:30 a.m. to 12:30 p.m. Bananas were placed out about every 2hr to account for the bananas that were removed by squirrels.
Figure 1. The Wilson Botanical Garden (light green) in Las Cruces Biological Station, Costa Rica. Each circle represents a distance of 25m from the established feeding station (red). The additional feeding stations are denoted in yellow. Brown squares represent buildings and dark green represents primary forest.
Behavioral Observations
Following the weeklong conditioning period, we monitored birds’ behavior at each feeding station between 5:30 a.m. and 12:30 p.m. from 12 July to 26 July 2013. We randomly selected the order in which we observed the stations, and each station was observed for one 30min period using visual observations or a Bell and Howell DNV900HD camera recorder (Bell and Howell, Durham, NC). Total observation time was 390min. During observations, we recorded each species that came to the station, the amount of time each bird spent on the station, and all displacement events, including which species was the displacer and which was the displacee. We considered a bird to be the displacer when it caused another bird to leave the feeding platform, either by non-physical intimidation or physical contact. The bird that left was the displacee. We used tallies of these observations to determine which species were higher on the dominance hierarchy (see analysis below).
Figure 2. Adjusted dominance scores (dominance score/adjusted dominance score) of 13 neotropical bird species, based on 232 interactions observed at feeding stations located around the Wilson Botanical Garden. Dominance scores were calculated as (# of times species displaced another species) – (# of times species was displaced). We adjusted the dominance scores for each species by the number of times that species was observed, so as not to over weigh common species.
Statistical Analyses
To determine the adjusted dominance score of each species we used the following equation: dominance score/Σ of sightings. The dominance score of a species was determined by subtracting the number of times individuals of the species were displaced from the number of times an individual displaced a bird of another species. To test for significant differences in the adjusted dominance scores of each species, we used a contingency table to show the frequency distribution. In order to determine whether there was a relationship between the adjusted dominance scores and species’ weights, we ran a linear regression. We obtained the average weight of each species by consulting A Guide to the Birds of Costa Rica (Stiles & Skutch, 1989). For statistical tests, we used a chi-square to test the significance of the interspecific interactions (Lebbin, 2008) and a non-parametric Kruskal-Wallis test to compare the total time spent on the feeding platforms by each species. It should be noted that although we treated each individual sighting as independent, the birds were not individually marked, so multiple visits by the same individuals could have violated this assumption.
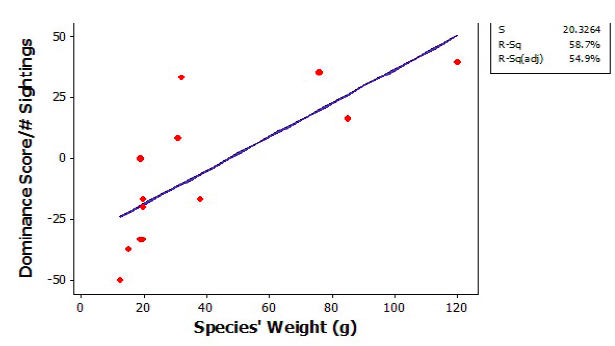
Figure 3. Linear regression of adjusted dominance scores (dominance score/adjusted dominance score) vs. weight (g) of 13 neotropical bird species.
RESULTS
Social Donimance Hierarchy
We observed 13 bird species and 232 interactions during 150min of video recordings and 240min of direct observation of the 13 banana feeding stations. We found strong evidence for an interspecific dominance hierarchy in these neotropical birds (X2 = 35.71; p < .001; df = 12; Figure 2). Blue-crowned motmots (Momotus momota) had the highest dominance score, while yellow-crowned euphonias (Euphonia luteicapilla) had the lowest (Figure 2). Blue-gray tanagers (Thraupis episcopus) had a dominance score that deviated from the other tanagers of a similar size (Figure 2). Despite this, the social hierarchy was consistent with body size. Dominance status generally correlated with body weight among species (linear regression r2= 0.587, Figure 3).
Time at Feeding Platforms
Time spent at the feeding platforms did not differ significantly between species, although the two largest species, the blue-crowned motmot and clay-colored thrush (Turdus grayi), spent more time on the platforms than the other species (H = 21.34; df = 12; p = 0.046; Figure 4).
Figure 4. Boxplots of time (min) spent on feeding platforms for each of 13 bird species, based on 390min of observation of 13 feeding platforms in the Wilson Botanical Garden, in Las Cruces Biological Station, Costa Rica.
DISCUSSION
Feeding Station Activity
The greatest bird activity occurred at the established feeding station in the nucleus of our study site, and thus a majority of the observations were recorded there. The interactions between bird species at this site likely revealed the patterns of a long-established dominance hierarchy, since the feeding station has been in use for 15 years. The temporary bamboo feeding stations we constructed required a greate r learning curve for some of the species to find and remember the locations of the individual stations. Silver-throated tanagers (Tangara icterocephala) were often observed at the new feeding stations earlier than most other species, and their presence at the feeding stations may have cued other species into the presence of a novel food source.
The relatively small size of the platforms (20 x 25cm) may have prevented active competition between some species. Indeed, mostly single individuals were observed at these stations, with few interactions (competitive or otherwise) with other birds. There were very few displacement events observed at these temporary feeding stations. Toward the end of the study, blue-crowned motmots were observed with increased frequency at these bamboo stations compared to the established feeding station, possibly preferring the lack of displacement interactions they would have to initiate.
Time at Feeding Platform
We hypothesized more subordinate species would spend less time feeding or waiting at the feeding stations in order to be more vigilant and less vulnerable to competing species and predators. We found no significant difference between species in the amount of time spent on the feeding stations. However, the top two dominant species, the blue-crowned motmot and clay-colored thrush, spent more time on the stations than the other species. Daily and Ehrlich (1994) found a significant negative correlation between the probability of a species being interrupted while foraging and its social dominance status. Our feeding stations were lower and more in the open than the trees where Daily and Ehrlich (1994) made their observations, perhaps making the birds feel more vulnerable to predators. This difference in location of the behavioral observations may explain why all the species we observed showed relatively short times spent on the platforms, as well as why we did not obtain the same results as Daily and Ehrlich (1994). This result is interesting because it suggests that dominance hierarchies can be malleable based on species’ perceived risk of predation.
Dominance Interactions
During periods of limited resource availability, the cost of displacement or defensive behaviors may be deemed necessary by some individuals (Case & Gilpin, 1974). However, some studies suggest that species high on the dominance hierarchy may display costly interference behaviors regardless of food stress (Rowell, 1974). We hypothesized that more dominant species would be larger, because the cost of being aggressive is less of an expense for those species. Our hypothesis was supported, and we found the most dominant species were the blue-crowned motmot, clay-colored thrush, blue-gray tanager and Bback-headed saltator (Saltator atriceps), while the least dominant were the tellow-crowned euphonia, thick-billed euphonia (Euphonia laniirostris), golden-hooded tanager (Thraupis larvata) and speckled tanager.
Though our results in dominance factors were similar, other aspects of our study differed from that of Daily and Ehrlich (1994), who found the most dominant species during the dry season at naturally fruiting trees were the buff-throated saltator (Saltator maximus), clay-colored thrush, Cherrie’s tanager (Ramphocelus passerine) and palm tanager (Thraupis palmarum), while the most subordinate species were the bayheaded Ttnager (Thraupis gyrola), golden-hooded tanager (Tangara larvata), thick-billed euphonia and Tennessee warbler (Vermivora peregrine). The differences we observed in the dominance hierarchy of Daily and Ehrlich (1994) may reflect a slightly different suite of species interacting during the wet season, in addition to our observations being made at novel, man-made food resources. Repeating our study in the dry season, and including both natural and man-made food sources, would shed light on whether these possible explanations are supported.
While there were occasional bouts of aggression between conspecifics, the great majority of competitive interactions were interspecific. Many of the top-tier dominant species, such as blue-crowned motmots, simultaneously displaced multiple birds at the feeding stations and monopolized the resources even when they were not exclusively feeding. Blue-gray tanagers also showed surprisingly high levels of dominance when compared to other birds of a similar weight, and often displaced tanagers of similar sizes. In this species and the closely-related palm tanager, body size seems to not be an important determinant of dominance level. The positions of these species in the dominance hierarchy may be influenced more by bill width or length (Daily & Ehrlich, 1994), and it would be interesting to include these measurements in future studies.
Conclusions
Dominance patterns are particularly interesting due to their application to a diverse range of topics in ecology, including food limitation, invasive species, extinction risk and forest regeneration (Daily & Ehrlich, 1994; Holway, 1999; French & Smith, 2005). For instance, as some species are removed from or introduced to a community, positions in a dominance hierarchy could potentially shift to accommodate the change, impacting patterns of seed dispersal and forest structure (Holway, 1999; French & Smith, 2005). By understanding which factors influence dominance in a social system, we can better determine which species will be more likely to adapt to a changing environment, which could be useful in designing conservation programs. For future studies, comparisons with long-term records of dominance status would be beneficial for gaining a more complete understanding of this complex behavioral system, such as we did here with our comparison to Daily and Ehrlich’s (1994) results. Other studies could include looking into the unusually high aggression observed in blue-gray tanagers to determine if this result was due to a small sample size, or a different cause.
ACKNOWLEDGEMENTS
We would like to thank Uakoko Chong for her help in the project design and implementation, as well as Joseph Jack, Melanie Kelliipuleole and Devan Tatemichi for helping us set up our feeding stations, which were made with the help of the Las Cruces maintenance crew and staff. We thank Brian O’Neil and Andrew Michelson for statistical advice, Wendy Townsend for her support and observations and Michael Breed for his help and suggestions. We also thank the Organization for Tropical Studies (OTS) and the National Science Foundation (NSF) for funding. B. Thornton thanks the College of Arts and Sciences at the University of Colorado Boulder for supplemental funding and especially Dr. Sue Lentz for her continued encouragement.
REFERENCES
Bernstein, I., & C. Ehardt. (1985). Intragroup agnostic behavior in rhesus monkeys (Macaca mulatta). International Journal of Primatology, 6(3), 209-226.
Case, T. J. & M. Gilpin, M. (1974). Interference Competition and Niche Theory.Proceedings of the National Academy of Sciences, 71(8), 3073-3077.
Chase, I. D. (1982). Dynamics of hierarchy formation: the sequential development of dominance relationships. Behaviour, 80(3-4), 218-240.
Daily, G. C. & P. R. Ehrlich, P. R. (1994). Influence of Social Status on Individual Foraging and Community Structure in a Bird Guild. Oecologia, 100(1-2), 153-165.
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour, 125(3), 283-313.
ArcGIS
(Version 9.3.1.) [Computer software]. Redlands, CA: ESRI.
French, A. R. & T. B. Smith, T. B. (2005). Importance of Body Size in Determining Dominance Hierarchies among Diverse Tropical Frugivores. Biotropica, 37(1), 96-101.
Hawley, P. H. (1999). The Ontogenesis of Social Dominance: A Strategy-Based Evolutionary Perspective. Developmental Review, 19(1), 97-132.
Holway, D. A. (1999), Competitive Mechanisms Underlying the Displacement of Native Ants by the Invasive Argentine Ant. Ecology Durham, 80(1), 238-251.
Lebbin, D. J. (2008). Aggressive interactions and preliminary evidence for reversed sexual dominance in Ramphocelus Tanagers. Ornitologia Neotropical, 19(3), 329-334.
Morse, D. H. (1974). Niche Breadth as a Function of Social Dominance. The American Naturalist, 818-830.
Pratt, T. K. (1984). Examples of Tropical Frugivores Defending Fruit-Bearing Plants. The Condor, 86(2), 123-9.
Rowell, T. E. (1966). Forest living baboons in Uganda. Journal of Zoology, 149(3), 344-364.
Rowell, T. E. (1974). The Concept of Social Dominance. Behavioral Biology, 11(2), 131-54.
Snow, D. W. (1981). Tropical Frugivorous Birds and their Food Plants: A World Survey. Biotropica, 13(1), 1-14.
Stiles, G. F. & Skutch, A. F. (1989). A Guide to the Birds of Costa Rica. Ithaca, NY:Cornell University Press.
Tilman, D. (2004). Niche Tradeoffs, Neutrality, and Community Structure: A Stochastic Theory of Resource Competition, Invasion, and Community Assembly. Proceedings of the National Academy of Sciences, 101(30), 10854-10861.
Vandermeer, J. H. (1972). Niche Theory. Annual Review of Ecology and Systematics, 107-132.