Authors: D. Adjaye-Gbewonyo, E. C. Quaye*, and D. A. Wubah**
Institution: Harvard University, *University of Cape Coast, **Virginia Polytechnic Institute and State University
Date: June 2010
Abstract
Three replicates were conducted simultaneously in separate blocks with daily applications of five treatments: a 1:8 P. guineense : distilled water stock solution, 60% and 80% dilutions of the stock, a 1:3:2 P. guineense : ethanol: distilled water solution, and a control of distilled water. Insect damage to leaves was recorded during application and for six days following the treatments to assess residual effects. On any given day, no significant difference among treatments was found in percentage or number of leaves damaged by insects or in any of the growth parameters. However, over all dates as a whole and considering the spraying and post-spraying periods separately, there was a significant difference among treatments for these parameters. Overall, the ethanol treatment had the lowest percentage for insect damage (39.118%), followed by the 80% aqueous extract (40.91%), 60% aqueous extract (44.97%), control (66.55%), and the stock aqueous extract (66.69%).
Keywords: Pest control, pest management, natural pesticides, Guinea pepper, insecticides, cow peas.
Introduction
Researchers in North America and around the world have been exploring natural alternatives to synthetic pesticides because of some of the risks associated with synthetic pesticides, such as environmental persistence or negative health effects related to their toxicity (Scott et al. 2005; Scott et al. 2004). According to E. C. Quaye (2006), scientists in Ghana are interested in following suit in order to reduce the usage of organochloride pesticides in local agriculture. Another motivation behind this interest in Ghana is the sheer cost of insecticides, which limits and even prevents many Ghanaian farmers from purchasing them to protect their crops. Thus, the development of safe and cost-effective insecticides using indigenous plants is being investigated by local researchers.
A native plant of Ghana and other parts of Africa, Piper guineense, which is sometimes referred to as Guinea pepper or West African black pepper, is a good candidate for an alternative insecticide for several reasons. Because it has traditionally been used as a spice and medicine, it has proven safe for humans (Scott et al. 2005). Additionally, many Piper species have been found to have insecticidal properties, especially P. nigrum, P. guineense, and P. tuberculatum (Scott et al. 2005). Responsible for the insecticidal properties of these species are secondary compounds that include neurotoxic piperamides and lignans (Scott et al. 2002; Scott et al. 2005). Piperine is the main amide active in P. guineense (Scott et al. 2004). In a study comparing the three Piper species listed above, P. guineense extracts had the lowest effective concentration for 50% mortality of Aedes atropalpus mosquito larvae, although the difference was not statistically significant (Scott et al. 2002). Further positive indication of P. guineense insecticidal activity is evident in several other studies where P. guineense powder, oil, and hexane and acetone extracts have been effective in causing mortality and reducing oviposition of various insects when applied to grains and crops such as maize or cowpea (Golob et al. 1999). In addition, another positive finding is that in comparisons of the toxic effects of the three aforementioned Piper species on non-target organisms, P. guineense was the least lethal to the earthworm (Scott et al. 2005).
Most research on P. guineense as an insecticide has been on the seeds, although Gbewonyo and Candy (1992) have found extracts from the male root to be active insecticides. However, previous work on P. guineense has generally been conducted in the laboratory setting or otherwise focused on observing insect toxicity directly rather than through pest activity on host plants. In this study, insecticidal activity is assessed in a more practical setting through damage to a crop grown in situ. Additionally, because of an eventual desire to develop an insecticide that can be easily and cheaply prepared by farmers themselves, this study focuses on aqueous extracts,which are less costly for farmers than solvents,and extracts of the whole seeds instead of isolated compounds. Although the active ingredients are not separated in the formulations in this study, the use of plant parts as a whole would also be more convenient for preparation by farmers. Furthermore, research has shown that extracts as a whole are less prone to develop resistance than isolated active compounds (Scott et al. 2004).
For application of the treatments, Vigna unguiculata, commonly known as the cowpea or black-eye bean, was chosen to represent a typical crop because it is indigenous to Africa and is the most diverse specifically in the West African savannah region; it also serves as a large source of protein in the African continent where the majority of the world's supply is produced (Madamba et al. 2006). Furthermore, the main challenge in cowpea production arises from pests and diseases, yet chemicals are not commonly used on cowpea because of the potential hazards, especially since the leaves are often eaten (Madamba et al. 2006; Kossou et al. 2004). Thus, cowpea serves as a model crop for examining natural insecticides, and, in fact, indigenous practices involving plant extracts are already in use by farmers to control cowpea pests according to a study in Benin (Kossou et al. 2004).
Materials and Methods
Plant Preparation
Figure 1: Field site at the University of Cape Coast near the botanical gardens. The image depicts the three blocks of beds where seedlings were planted and treatments applied.
Fifteen beds were prepared in three replicate blocks,five beds to a block corresponding to the five treatments,in the field near the botanical garden behind the science faculty of the University of Cape Coast (Figure 1).
Beds were around 260 cm by 80 cm each. The soil in this area is described as lateritic, as it contains high iron and aluminum content, and it is sandy and fine with top soil about a third of a meter deep (Central Regional Coordinating Council 2006). Dry cowpea seeds were obtained from an assistant at the university's herbarium and soaked overnight, and on July 11, 2006, three seeds were sown in each of three holes per bed about 80 cm apart. The holes were then watered, and following germination,which occurred within three to five days,seedlings were thinned to one plant per hole on July 17, 2006. Weather conditions throughout the study period were mostly sunny and breezy with daytime temperatures ranging from the upper 70s to mid 80s Fahrenheit. Rainfall occurred on a number of days during the spraying and post-spraying period, specifically at the end of July and in early August.
Extraction
P. guineense seeds were purchased from a local market in Cape Coast, Ghana. For the aqueous extract, 250g of seeds were ground in a blender and mortar and pestle. A total of 2L of distilled water was added and the solution filtered to create the stock solution. 100mL of 80% and 60% solutions were made by further dilutions of the stock solution with distilled water in the appropriate proportions (80 mL of the stock solution with 20mL of distilled water and 60 mL of the stock with 40mL distilled water, respectively).
For the solvent extract, 100g of P. guineense seeds were ground in the same manner as the aqueous solutions and mixed with 300mL of 96% ethanol. The solution was filtered and diluted with 200mL distilled water. All solutions were stored in a refrigerator at 0oC.
Field Treatments
To monitor the growth of the cowpea seedlings, beginning 6 days after planting,at which time insect damage had already begun,the stem height from the soil level to the first leaves and the length and the breadth of each of the first two leaves were measured using a ruler. These measurements were repeated every other day along with a leaf count of additional leaves. Plants were watered regularly by the garden staff.
Figure 2: Diagram of field layout illustrating each bed and its randomized treatment. Each block contains the following treatments: stock aqueous solution, 80% stock solution, 60% stock solution, ethanol extract, and control.
Each of the five beds within the three blocks was randomly assigned one of the five treatments (stock aqueous solution, 80% aqueous solution, 60% aqueous solution, ethanol extract, control of distilled water). An illustration of the treatment layout is given in Figure 2. Beginning with the control treatments and continuing in order of increasing concentration, the foliage of each seedling was sprayed about 7 sprays of the assigned treatment using a Harry Brand Sprayer spray gun. The spray gun was rinsed with distilled water in between the aqueous and ethanol extract applications. Spraying was done daily in the afternoon for five days.
Prior to spraying, each seedling was examined and the number and proportion of leaves damaged by insects was recorded. Leaves were considered to have insect damage if they showed signs of chewing or contained holes (Figure 3). Holes with the leaf cuticle remaining,some of which later dried out, leaving brown spots,were also included as insect damage because the underside appeared to be rough as though chewed by a small insect but without complete penetration. Symptoms that were more indicative of disease and deformities,such as discoloration, bumps, and indentations with smooth edges as opposed to the rough edges associated with chewing,were not included in the count. The count was done daily during the spraying period and daily following the spraying period from July 30 through August 4, 2006 to assess residual effects.
Statistics
Figure 3: Vigna unguiculata (cowpea) seedling with insect pest damage.
Field data was analyzed with MINITAB software using a balanced analysis of variance (ANOVA) for a randomized complete block design with multiple observations. Where data was missing due to failed germination or death of a plant, the mean value for the corresponding bed was used in order to balance the data without affecting the overall mean. Pairwise comparisons of means were conducted using Fisher's least significant difference (LSD) method.
Results
Two of the insect pests observed on the cowpea plants were identified as Empoasca spp. , the leafhopper, and Zonocerus variegatus, the variegated grasshopper. Multiple leafhoppers were seen resting on the leaves of the plants although actual feeding was not observed. However, leafhoppers are recognized as one of the common pests of the cowpea (Madamba et al. 2006). One Z. variegatus larva was observed August 1-4 2006 feeding on the first and second plants of the ethanol treatment in Block C (Figure 4).
In general, there appeared to be less insect activity on the bottom two oldest leaves of the seedlings. Similarly, the newest group of leaves, which naturally have small surface areas and may thus not be as likely to be attacked by insects, tended to have less or no insect damage.
Figure 4: Zonocerus variegatus (variegated grasshopper) feeding on a cowpea seedling.
The results of the statistical analysis indicated a significant difference in treatment effects on the number of leaves per plant damaged by insects over all dates, with a P-value of 0.000. In order of increasing number of damaged leaves, the treatments ranked, 60% aqueous, ethanol, 80% aqueous, stock, control. In terms of percentage of damaged leaves, which is more useful than the absolute number as total number of leaves per plant may differ, there also was a significant difference in treatments (P=0.000) for the mean percentage of total leaves damaged by insects when all dates were considered. Overall, the ethanol treatment had the lowest percentage, with a value of 39.11%, followed by the 80% aqueous extract (40.91%), 60% aqueous extract (44.97%), control (66.55%), and the stock aqueous extract (66.69%). The Fisher's LSD comparisons indicated that the significant difference was between the following pairs: control and ethanol, control and 80%, control and 60%, 60% and ethanol, 60% and stock, 80% and stock, and stock and ethanol.
In order to determine whether the significant difference was a result of the period when spraying occurred or the post-spraying period, the data was separated into the two periods. In both periods, there was still a significant difference between treatments for insect damage (number and percentage of damaged leaves), all with a P-value of 0.000. For the spraying period overall, the percent damage for treatments in increasing order was 80% aqueous (33.90%), ethanol (42.29%), 60% aqueous (44.96%), control (65.24%), and stock aqueous (69.12%). Differences were significant between all pairs except the control and stock, 60% and ethanol, and 80% and ethanol. The results for the period following spraying were ethanol (36.46%), 60% aqueous (44.97%), 80% aqueous (46.76%), stock (64.65%), and control (67.65%), with significant differences between all pairs except the control and stock and the 60% and 80% aqueous solutions.
However, on any given day, there was no significant difference between treatment means for insect damage (P=0.976 for number of damaged leaves and P=0.945 for percentage of total leaves damaged for analyses of treatments by date). Figure 5 illustrates the damage over time.
Figure 5: Percentage of leaves containing insect damage by treatment over time. The percentage includes seedlings in all three replicates for each treatment and is based on daily observation. Days 1 through 5 (July 24-28) refer to days on which spraying occurred while days 7 through 12 (July 30-August 4) were observed in the period following spraying. Observations were not made on day 6 (July 29).
In terms of growth parameters, there was a significant effect of treatments overall on the total number of leaves on the plants (P=0.000). In descending order, the mean number of leaves for each treatment over all dates was: control (13.1), ethanol (11.4), stock (11.2), 80% (10.9), 60% (8.9). Differences were significant between the control and every other treatment and between the 60% and each of the following: ethanol, 80%, and stock. For the two separate periods, the difference in number of leaves remained significant in each period (P=0.000) with control > stock aqueous > ethanol > 80% > 60% during the spraying period as a whole. Pairwise analysis indicated that each treatment was significantly different from every other except the stock and ethanol. The results for total number of leaves during the post-spraying period were: control > ethanol > stock aqueous >80% > 60%, with all treatments differing significantly from all others except for the following pairs: 80% and stock, stock and ethanol, and 80% and ethanol. Again, on a daily basis, however, there were no significant treatment effects on the total number of leaves.
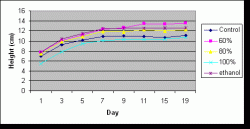
Figure 6: Average (mean) height of seedlings by treatment over time. Measurements taken on days 1 through 7 (July 17-23) preceded application of the treatments. Days 9 through 11 (July 25-27) were within the spraying period, and measurements on days 15 through 19 (July 31-August 4) followed the spraying period. Height measurements were made every other day (excluding day 13 (July 29) and day 17 (August 2) due to rain).
Similarly, there was a significant difference in treatment effects overall on mean height (P=0.000) but no significant difference of treatment effects on height when analyzed by date (P=1.000). Height was not analyzed by the two separate periods because of time constraints. The mean heights for treatments over all days in increasing order was stock (9.311 cm), control (10.128 cm), 80% (11.052 cm), ethanol (11.542 cm), and 60% (11.810 cm). The only pairs that were not significantly different from each other were 60% and ethanol and 80% and ethanol. Figure 6 illustrates the average change in height with time for each treatment.
Discussion
Although the replicates were separated into blocks, because there were no known differences among the blocks,which were located beside each other and appeared to be homogeneous,block effects were not expected. For the percentage of damaged leaves, which was the principal outcome, this was the case as the statistical analysis found no significant block effects as a whole or in the separate periods. This would indicate that running the analysis as a completely randomized design without blocking would have been sufficient. However, for other factors (height, total number of leaves, and number of damaged leaves), the ANOVA results did indicate significant differences between blocks for the study periods. An explanation for differences between blocks cannot be given, but differences do not appear to have been systematic. Furthermore, maintaining the blocking in the analysis accounted for any of these differences that were unrelated to the treatments.
In terms of growth parameters, the control of distilled water seemed to remain the group with the most number of leaves. This would be expected if treatments affected growth; however the order of the treatments for total leaf number and the different resulting order for the other growth parameter, height, are not consistent with growth effects related to the dose of the treatments. Also, two transplants were made from additional seedlings into two holes that did not germinate, and these transplants did not grow as tall as the other plants. This could have affected growth results but should not have had a large influence overall considering the total number of remaining plants.
Analysis over Time
The effect of the date was significant on height, total number of leaves, and number of damaged leaves. This was expected for the growth parameters because the number of leaves and the height should increase significantly with time. The resulting significance of time on growth could indicate that the treatments did not affect or stunt the growth of the plants. The number of damaged leaves also increased significantly with time. However, if the treatments were functioning as insecticides, one would expect the number of damaged leaves to stay fairly constant and not increase significantly with time for most treatments besides the control, at least during the spraying period. When separated into the two periods, though, the date did not significantly affect the number of damaged leaves during the spraying period as a whole and considering all treatments (P=0.133). So, the significance in date on number of damaged leaves resulted from the period following spraying (P=0.000), which suggests that there were no significant residual effects of the treatments. Residual effects were not expected because during the second period there was a lot of rainfall which would have rinsed off any remaining treatment, while during the spray period there was little rainfall.
On the other hand, the percentage of total leaves with insect damage was not significantly affected by the date overall. When split into the separate periods, the date is clearly insignificant during the week of spraying (P=0.998), but it is almost significant during the post-spraying period (P=0.062). This could be similarly due to the lack of residual treatment effects which would result in significant increases in the percentage when spraying ceased. However, one might expect significant decreases in the proportion of leaves attacked by pests during the spraying period if treatments were effective, which was not the case. Figure 5 also illustrates no clear trend in proportion of pest damage during the first five days of spraying, which seems to indicate that the treatments had weak insecticidal efficacy.
Effects of Treatment Concentration
Overall, the treatments ranked in order of increasing percent insect damage are: ethanol, 80% aqueous, 60% aqueous, control, and stock aqueous as stated above. One would expect the ranking to correspond to the concentration of P. guineense and thus be: ethanol, stock, 80%, 60%, control. There does not appear to be a logical reason for the observed ordering besides the fact that the ethanol treatment had the least percent damage. Moreover, as seen in the results above, the ranking is not identical when the data is separated into the two periods. These results do not seem to suggest a dose-response relationship or that the amount of insect damage was related to the treatment concentration.
It is especially unexpected that the stock, which is the most concentrated aqueous solution, consistently had one of the highest rates of insect damage. One possible explanation could be that P. guineense functions through insect mortality and not repellence; so insects would still feed on the plant and would only be affected afterwards. However, this would not agree with previous research where P. guineense exhibited repellent properties against the red flour beetle on paper discs (Scott et al. 2004).
A possible contributing factor to results that do not follow expected patterns may be because applications of the treatments were made on the same day and not the same stage of growth and damage. Thus, initial differences in the amount of damage could have affected the outcome. For instance, on the day that application of the treatments began, ethanol and 80% aqueous tied for the lowest percentage of leaves damaged by insects (40.28%), followed by 60% aqueous (45.71%), control (64.51%), and stock aqueous (66.67%). Furthermore, there were already initial significant differences between the control and ethanol, control and 80%, control and 60%, 60% and stock, 80% and stock, and stock and ethanol. These initial rankings correspond more closely with the overall ranking, which could indicate that pre-existing differences in damage to plants of different treatments affected the outcome; but,as evidenced by the crossing over of lines in the chart in Figure 5,this ranking is not consistent and varies depending on the date. These inconsistencies explain the lack of significant differences in treatment effects by date as opposed to as a whole.
Other reasons for the failure to observe consistent and conclusive effects of the treatments could be low concentrations of the P. guineense extract in general. However, in previous studies, a concentration as low as less than 0.1% of P. guineensewas effective as an insecticide, although in that instance it was applied directly to the larvae (Scott et al. 2004) Moreover, though unlikely, minor differences in the amount of extract sprayed on plant surfaces could have affected the results. While plants were given equal number of sprays in order to approach equal volume of extract applied, surface areas of the seedlings could have differed slightly and resulted in unequal distribution of the treatment. Another method used in other studies that might better account for this is spraying until saturation or runoff rather than controlling the amount applied (Scott et al. 2004). Alternatively, the extracts could have lost potency because of degradation of the active compounds. Photodegradation of piperamides has been previously observed in studies of Piper species. Scott et al. (2004) noted that after six hours in sunlight, the active ingredient in P. guineense had virtually completely degraded. Plants in this study were not protected by shade and could have thus been affected by photodegradation. There may also have been some error in measurements of growth or the classification of insect damage. However, as mentioned in the methods, consistent criteria were used throughout.
Therefore, because of some initial differences in parameters, the lack of a dose-response relationship in the overall ranking of treatments by percent insect damage, and inconsistencies in results over time, definite conclusions on the effects of Piper guineense extracts on insect damage and growth of the cowpea plant in its vegetative stage cannot be drawn from this study alone. Further studies should take into account or control for initial differences in damage. For instance, in examining the repellency of Piper nigrum, Scott et al. (2004) normalized the number of leaves on each plant by leaving only 10 healthy leaves.
In order to obtain better results, extended time is also needed to repeat the application process to collect more data beyond the vegetative stage through the flowering and fruiting stage of the plants. In fact, studies have shown that the best way to protect yield of cowpea plants is by applying insecticide to pests of the flowers and pods. (Kyamanywa 1996). Further suggestions include soil analysis to assess effects of soil characteristics on the results and monitor possible uptake of the extracts through the soil; the use of more concentrated extracts; and testing other solvents instead of ethanol to reduce the toxic effects since plants treated with the ethanol extract quickly showed signs of toxicity,such as wilting leaves or dried patches, reddish brown spots, and eventually more deformities and stunted growth in new leaves. However, in the Scott et al. (2004) study, the control plant was also sprayed with an ethanol-distilled water solution and no effects were mentioned. The toxicity could also perhaps be due to the fact that the P. guineense extract in ethanol was more concentrated compared to the aqueous extracts. For example, in the same Scott et al. (2004) study, 1% P. nigrum spray was toxic to lily plants; though in another field study 2-4% P. nigrum was not toxic to turfgrass (Scott et al. 2005).
Further study should also include comparisons with conventional insecticides as well as with other more known botanical insecticides such as neem. If possible, combining P. guineense with other botanical insecticides can also be investigated. Additionally, if the eventual goal is for P. guineense aqueous extracts to be made by local farmers in Ghana, studies should be conducted within a practical farm setting to take into account other factors that could affect results. These factors might include the surrounding land uses and differences in farmer preparation, such as alternative water sources, as distilled water is not likely to be available to most farmers.
Acknowledgments
This project was funded by the National Science Foundation International Research Experience for Undergraduates grant (#0452779) to DAW. We thank the laboratory staff in the Department of Botany at the University of Cape Coast, Ghana for their assistance in carrying out this project.
------
References
Central Regional Coordinating Council (2006) Cape Coast Municipality. Retrieved May 2, 2010 from http://www.centralregion.gov.gh/district/cape%20coast.php
Gbewonyo, W. S. K. and D.J. Candy (1992) Separation of insecticidal components from an extract of the roots of male P. guineense (West African black pepper) by gas chromatography. Toxicon 30, 1037-1042.
Golob, P. et al. (1999) The Use of Spices and Medicinals as Bioactive Protectants for Grains. Rome: Food and Agriculture Organization of the United Nations. Retrieved August 9, 2006, from http://www.fao.org/docrep/x2230e/x2230e11.htm
Irvine, F.R. (1961) Woody Plants of Ghana: With Special Reference to Their Uses. London: Oxford University Press.
Kossou, D. K. et al. (2001) Indigenous cowpea production and protection practices in Benin. Insect Science Application 21, 123-132.
Kyamanywa, S. (1996) Influence of time of insecticide application on control of insect pests of cowpea and grain yield of cowpea at Mtwapa, Coastal province of Kenya. African Crop Science Journal 4, 373-382.
Madamba, R. et al. (2006) Vigna unguiculata (L.) Walp. In Brink, M. & Belay, G. (Editors). Plant Resources of Tropical Africa 1. Cereals and pulses. PROTA Foundation, Wageningen, Netherlands/ Backhuys Publishers, Leiden, Netherlands/CTA, Wageningen, Netherlands. Pp. 221-229.
Scott, I. M. et al. (2005) Efficacy of botanical insecticides from Piper species (Piperaceae) extracts for control of European chafer (Coleoptera: Scarabaeidae). Journal of Economic Entomology 98, 845-855.
Scott, I.M., et al. (2004) Efficacy of Piper (Piperaceae) extracts for control of common home and garden insect pests. Journal of Economic Entomology 97, 1390-1403.
Scott, I.M., et al. (2002) Insecticidal activity of Piper tuberculatum Jacq. extracts: synergistic interaction of piperamides. Agricultural and Forest Entomology 4, 137-144.
Quaye, E.C. (2006) Personal communication with Dr. Quaye regarding pesticide use for farming in Ghana. (July 4, 2006, Department of Botany, University of Cape Coast, Ghana).