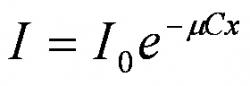Authors: Bob Dawe, Terri Erickson
Institution: Laboratory for Product and Process Design, University of Illinois at Chicago
Date: May 2008
ABSTRACT
Targeted delivery of large therapeutic macromolecules to desired locations inside the brain via systemic delivery is hampered by the function of the blood-brain barrier (BBB), which is formed by tight junctions of the epithelium that lines capillaries in the brain. Recently, direct injection methods such as the so-called Convection-enhanced delivery (CED) are pursued to effectively bypass this vascular barrier and using an infusion catheter whose tip is placed close to the target site. In this technique, a cannula is inserted directly into the area of the brain to be treated, and the therapeutic agent is delivered through the cannula via bulk flow, circumventing the BBB. The efficacy of direct injection methods is assessed by achievable penetration depth and drug distribution volume defined as the region of the brain dosed above a certain therapeutic concentration threshold. In the scope of this paper, the threshold was set at 10% of the inlet drug concentration.
In this paper, in vitro investigations using surrogate brain gels (agarose) were conducted as a means of developing a standardized protocol for investigating the role of catheter design and infusion parameters that maximize distribution volumes in convection-enhanced delivery method.
Injection of two visible marker dyes: Trypan blue and Bromophenol blue of varying molecular weights were infused at various flow rates (0.5-5 μl/min) into agarose gel brain phantoms under observation to gather quantitative data regarding the distribution of therapeutics in the brain following CED. Computer models of infusion into the brain were also created to predict patterns of drug distribution. The penetration depth, volume of distribution, and concentration profile of this dye is assessed for a variety of infusion policies.
Achievable volumes of distribution increased with convection-enhancement than that achievable by diffusion alone. In addition, a smaller cannula diameter and smaller weight species (bromophenol blue) produced larger penetration depths and treatment volumes.
Allowing the cannula to set into the gel, a method that mimics the healing of brain tissue around an inserted catheter resulted in minimal leakback along the catheter and successful infusion to the target area when flow rates were lower than 5 l/min. At flow rates higher than this, infusion patterns were unpredictable with extensive backflow (reflux) along the catheter shaft. Inserting the cannula into solidified agarose, injuring the gel, is relevant for cases where infusion through a catheter into the brain begins without allowing for healing time. This case resulted in reflux back up the cannula in every trial, though reflux distance was decreased with smaller diameter cannulas and lower flow rates.
INTRODUCTION
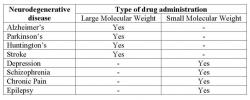
Neurodegenerative diseases of the brain such as Parkinson's and Alzheimer's disease are a critical medical issue that requires swift, efficient treatment. About ten million people in the United States suffer from diseases of the central nervous system, specifically dementia and stroke (Thorne and Frey 2001). It is known that the blood-brain barrier prevents delivery of large molecular weight drugs in reaching target regions when administered systemically (Lieberman et al. 1995; Pardridge 1999). CNS diseases that require large molecule drug therapy, listed in Table 1 are unable to pass through the blood-brain barrier because of their size (Pardridge 2005). Many therapeutic molecules that would be used to treat various neurodegenerative diseases fall into this category. Therefore, these therapeutic agents cannot be administered systemically (intravenously through the blood) because the epithelium that lines capillaries in the brain prevents them from migrating out of the blood and into the brain. Even if the blood-brain barrier could be disrupted to selectively allow these molecules to cross it, systemic drug delivery precludes the targeting of specific areas of the brain for treatment, while endorsing blanket treatment of the entire brain. As a result, direct injection with convection-enhanced delivery (CED) is necessary to bypass the blood-brain barrier (BBB) and reach the diseased areas of the brain (Lonser et al. 2007; Morrison et al. 1994).
Convection enhanced delivery is carried out by drilling a bur hole in the skull and inserting a catheter into the brain. Based on medical constraints, the infusion catheter tip is placed near the target region for optimal drug delivery (Morrison et al. 1994; 1999; Chen et al. 1997; Raghavan et al. 2000; Linninger et al. 2008; Somayaji et al. 2008). Transport of therapeutic agents is primarily due to diffusion with convection-enhancement which helps to overcome the supposedly low effective drug diffusivity in tissue. Therefore, bulk flow of the infusate out of the catheter will allow for much larger treatment volumes than with diffusion only. Delivery techniques would require less time and therefore reduce the occurrence of negative feedback post treatment. Procedures involving CED will allow the administration of therapeutic agents to be more effective in terms of treating the regions of interest with more potent levels. Though convection-enhancement can achieve wider drug distribution in the brain, proper choice of infusion parameters and catheter design impact the pattern and achievable volume of drug distribution defined by a therapeutic threshold. Optimal choice of infusion parameters such as infusate flow rate, drug dilution and typical catheter design parameters such as diameter, port configuration (single or multiple), attack angle, placement with respect to the target anatomy play an important role in the achieving desired distribution volumes (Chen et al. 1999).
Equation 1 1510
After stereotactically placing the infusion catheter near the target site, simple infusion is executed after the brain has healed (also referred to as preinfusion sealing time by some authors) around the catheter thus in a manner sealing the catheter. Another phenomenon that occurs with high infusion rates, typically more than 5µl/min, is reflux or backflow of the infusate along the catheter shaft (Morrison et al. 1999). This occurs when infusion is executed before the brain is allowed to heal. The injury is also caused by the insertion of the catheter creates space where the drug can flow back up the catheter (Raghavan et al. 2006). Reflux of the drug back up and around the shaft of the catheter is diminished when the tissue is allowed to heal around the catheter before infusion. In recent publications, there are two forms of reflux defined. Krauze et al. devised a cannula composed of fused silica tubing glued into a 27G needle to create a step-design which prevented reflux in agarose gels, rat brain and monkey brain tissue at all flow rates tested (Krauze et al. 2005).
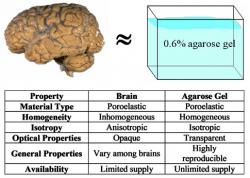
Figure 1: Comparison of brain and agarose gel properties. The agarose gel is a relatively inexpensive brain surrogate whose properties closely match with that of the brain tissue (Chen et al. 2002; 2004)
In addition, tissue porosity and tortuosity are two major factors that can cause the infusate's effective diffusion coefficient (in brain tissue) to be smaller than its bulk diffusion coefficient (Saltzman et al. 1991; Nicholson et al. 2001). This reduced diffusivity in porous media, which is due to the effects of porosity and tortuosity, is one reason why convection must be used in order to effectively deliver therapeutic agents to the brain. A tissue having a low porosity is said to have a small void fraction. That is, the tissue is composed mostly of solid fibers with very little fluid in the small fraction of space between the fibers. For the most part, the infusate cannot diffuse through the solid fibers; it can only occupy the interstitial fluid. Therefore, the low porosity of the tissue contributes to the enhancement of drag on the infusate molecules, since the molecules have to squeeze through small fluid channels and bump up against the solid fibers in order to diffuse. In general, a lower porosity causes the effective diffusion coefficient to be lower as well.
Figure 2: Schematic of the experimental setup. The main components are: a Harvard apparatus syringe pump, a stereotactic frame, a fluorescent light box to backlight the transparent gel as infusion is carried out. A digital camera connected to a PC to take snapshots of the dye distribution pattern at regular intervals and store the photographs to a hard disk
Outline
In this paper, we systematically investigate the role of catheter design and infusion parameters that maximize the achievable distribution volume (treatment volume) involved in convection enhanced delivery. However, in order to achieve these goals, an appropriate brain surrogate must be established to carry out preliminary experiments and achieve reproducible results. In our experiments, we studied the transport of two dyes: trypan blue and bromophenol blue in a relatively inexpensive agarose gel brain tissue surrogate whose properties closely match with that of the brain tissue (Chen et al. 2002; 2004) as given in Figure 1. Details on experimental procedure are discussed next.
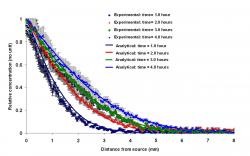
Figure 3: Diffusion of trypan blue dye through 0.6% agarose gel at room temperature. The reservoir of dye was at location 0 mm. Concentration profiles were obtained at 1, 2, 3, and 4 hours after the beginning of the experiment (dots with measurement error bars). Solutions of the one-dimensional diffusion equation are represented by smoothed lines. An effective diffusion coefficient of 2.8 × 10-10 m2/s was used to fit the theoretical concentration profiles to the experimental data
MATERIALS AND METHODS
In vitro Agarose Gel Models: Why use Agarose Gels?
For in vitro infusion studies, agarose gel was chosen to serve as surrogate brain tissue. Justifications for this choice of material are summarized in Figure 1. First, both brain tissue and agarose gel can both be considered poroelastic materials. Although overall the brain is inhomogeneous and anisotropic in its composition, localized regions of gray matter can be largely homogeneous and isotropic, like agarose gel. Furthermore, the homogeneity and isotropy of agarose gel makes it the most basic test bed with which to investigate CED. The transparency of the gel allows for acquisition of data using optical methods, rather than expensive MR or radiography. Agarose gel allows for the study of infusion at a basic level, allowing for reproducibility and perfection of methods thus allowing the prediction of infusion patterns with confidence before moving on to animal tissue experiments.
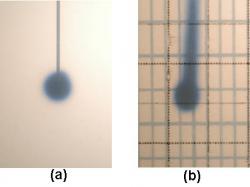
Figure 4: Photographs of convection enhanced infusion trials. The cannula in (a) was inserted into the solidified gel and hence shows no backflow or reflux. In frame (b), the cannula in the gel was not casted before infusion started and hence shows extensive reflux along the catheter shaft
Experimental Set-Up
This study was conducted in two parts, to investigate dye infusion into agarose gel with and without reflux. Both sets of experiments required surrogate brain casts made of 0.6% agarose gel and cast in 15 × 7.5 × 0.3175 cm (W × H × D) glass boxes, which were separated into three sections, each 4.0 cm wide, that could be used for different trials. For reflux-free trials, the cannula was placed in the agarose gel before it was allowed to set, whereas for reflux trials, the cannula was inserted into the gel after it solidified. Trypan blue and Bromophenol blue dyes were used in all experimental trials. A fluorescent light box was used to backlight the transparent gel as infusion was carried out. A digital camera (Nikon D100) on a tripod connected to a PC was used to take snapshots of the dye distribution pattern at regular intervals and store the photographs to a hard disk for later analysis. A full schematic of the set up can be seen in Figure 2.
Figure 5: The effect of infusate molecule size on transport in porous media. Diffusion of bromophenol blue, a smaller molecule, was more extensive than that of trypan blue, a slightly larger molecule. The CED parameters were identical for the two trials: 1.0 μl/min through a 27-gauge cannula for 30 minutes, followed by diffusion for 1 hour
Preparation of 0.6% Agarose Gel
Surrogate brain models, 0.6% agarose gel, were prepared by mixing 6 g of dry agarose (Fisher Biotech) with 9g NaCl (Sigma) in 1 L of distilled water. This mixture was autoclaved to 121° C to facilitate complete dissolution of the agarose, and then placed in a 49° C water bath for slow cooling to a few degrees above gelling temperature. Upon cooling to 49° C, 10mL of the liquid agarose was injected using a syringe and 23 3/4G needle into the glass box, in which the cannula was previously inserted by the aide of the stereotactic frame. Cooling was allowed and gelling occurred around 40° C. Throughout the gel preparation process, efforts were made to minimize evaporation. The unused portion of liquid agarose was stored in a sealed glass bottle in a water bath for no more than one week.
Preparation of the Dyes
Trypan 0.02% blue dye (molecular weight, M = 961 Kg/Kmol) and 0.25% Bromophenol blue dye (molecular weight, M = 669 Kg/Kmol) were used in the trials. Bromophenol blue (Sigma) is a blue-black, odorless powder with melting point of 273ºC. Its chemical formula is C19H10Br4O5S, and it is water-soluble. Dye was prepared by mixing 0.25 grams Bromophenol blue (BPB) powder with 100ml of distilled water for a 0.25% concentration. The solution was mixed using a vortex and stored at room temperature. The dyes were infused into each brain phantom via the cannula embedded in the gel so as to minimize infusate backflow along the catheter shaft. Each experiment was repeated three times to estimate measurement error.
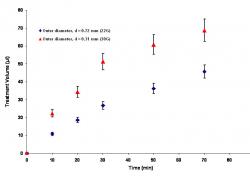
Figure 6: The effect of cannula diameter on transport in porous media during CED. During 30 minutes of infusion at 1.0 μl/min, the treatment volume increased most rapidly when the smallest cannula, a 30-gauge, was used. The larger treatment volume obtained with the 30-gauge cannula persisted even after another hour, during which time no infusion took place
Figure 7: Representative concentration map and concentration profiles derived from experimental data. In (a), a typical concentration map is displayed and the pixel row from which a concentration profile was extracted is highlighted. In (b), the concentration profiles are shown at 20-minutes intervals during convection-enhanced delivery. The concentration profiles during diffusion only (following 60 minutes of CED) are shown in (c). Times shown in (c) are post-infusion times
Syringe and Pump Set-Up
A 10 ml BD plastic syringe was filled with 0.25% BPB dye and attached to a cannula via polyethylene tubing. This syringe was then placed into a Harvard Apparatus syringe pump so that precise infusion rates could be set. The cannula was attached to a stereotactic frame, which was used to lower or lift the cannula in and out of the agarose gel. Efforts to minimize the variant parameters allowed for control and reproducibility of the trials. The experimental set-up allowed for reproducibility with little to no variation. Parameters under investigation were cannula size and flow rate. The volume injected was held constant as to provide a baseline for comparison. Infusion time was adjusted respective to the flow rate while diffusion time was held at a constant hour with images taken every twenty minutes.
Table 1: List of neurodegenerative diseases and their therapy (Pardridge 2005)
Concentration data from images
Matlab Image Processing toolbox is used to determine the penetration depth and volume of distribution for each infusion trial. Dye concentration as a function of position can be obtained using one of three methods. The first method involves freezing the gel, then cutting it into thin slices for layer-by-layer concentration analysis. The second method makes use of the assumption that the dye distributions were radial symmetric to digitally reconstruct slices of the dye distribution with computed tomography (CT). In the third method, the gel is infused with an MRI-tagged substance and imaged in an MRI scanner.
In the scope of this paper, we relied on the second method and Beer-Lambert law given in equation (1) was deployed to convert pixel intensity of the image to dye concentration. This law describes how light is attenuated as it passes through a semi-translucent substance, trypan blue dye in this case. In equation (1), I, is the intensity of the light after it has passed through the substance in question, I0 is the initial light intensity before it passes through the substance, and μ, C, and x are the molar attenuation coefficient, concentration, and thickness of the substance, respectively.
RESULTS
This section will discuss the simple infusion of trypan blue dye into agarose gel phantoms. Quantification of reflux or backflow is not considered here and is reserved for future work. Matlab image processing toolbox is used to quantify penetration depth and distribution or treatment volume from the two-dimensional images recorded in the camera. The advantage of CED against diffusive transport in effective transport of the dye in the porous gel is also reported in this study. The unknown effective diffusivity of trypan blue in agarose gel has been determined from diffusion experiments via trial and error. In addition, the effect of cannula diameter and molecular weight of the dye are investigated. The transparent gel model of the brain and our system for the optical determination of dye concentration allowed us to quantitatively investigate several phenomena related to CED. The effects of diffusion and convection during CED were compared by varying infusion parameters such as cannula diameter. The resulting dye distributions were quantified.
Diffusion Experiments.
Diffusion of trypan blue dye through 0.6% agarose gel at room temperature is illustrated by Figure 3. The dye was allowed to diffuse from a reservoir located at the
0-mm position. The dots in the graph represent the experimentally-derived dye concentration profiles at 1, 2, 3, and 4 hours after the start of the experiment. Each trial was repeated three times to calculate the measurement error. The smoothed lines represent solutions to the one-dimensional convective diffusion equation which is in satisfactory agreement with the measurement error. In this fitting procedure, convection and reaction were assumed to be totally absent. The effective diffusivity of trypan blue was determined at, 2.8 × 10-10 m2/s to fit the theoretical concentration profiles to the experimental data.
Convection-Enhanced Delivery of Trypan Blue Dye
Figure 4a depicts dye distribution in an ideal situation without leak-back which is typical at very low flow rates. However, in the range of infusate flow rates typical in clinical practice (up to 9 µl/min), the most qualitatively observable result has been the presence or lack of reflux, or the leaking of dye back along the cannula shaft toward the surface of the gel. This reflux of dye causes a substantial, yet unknown decrease in the amount of dye that spreads radially from the infusion site, shown in Figure 4b. Without knowing the exact volume of dye that leaks back along the cannula, it will be difficult to assess the convection and diffusion of dye away from the infusion site. It seems that the easiest way to quantify reflux is to make it zero; that is, eliminate reflux completely. Thus, the early part of this study focused on finding a way to prevent dye reflux along the cannula.
Casting the cannula in the gel prior to gelling prevented the damage to the gel caused by stereotactic insertion of the cannula. Using this method, a tight seal between the gel and cannula is achieved, thus making it difficult for dye to leak back. Experimental results revealed no evidence of reflux when the cannula was cast in the gel, as illustrated in Figure 4a This cast in method will be used to avoid reflux in future experiments.
Effect of Molecular Weight
Bromophenol blue dye, molecular weight 669 Kg/Kmol, and Trypan blue dye, molecular weight 961 Kg/Kmol, were delivered into the agarose gel using identical infusion policies. The resulting treatment volumes over time are shown for each infusate in Figure 5. After 30 minutes of infusion at 1.0 μl/min followed by 1 hour of diffusion only, the final treatment volume for Bromophenol blue, the smaller molecule, was nearly four times that of Trypan blue, the larger molecule. The penetration depth of Bromophenol blue was about 5 mm, twice that achieved in trials involving Trypan blue dye.
Effect of Catheter Diamater
The diameter of the cannula used for infusion also affected the treatment volume. Figure 6 shows that the treatment volume increased most rapidly when the smallest of three cannulas was used for infusion. After 30 minutes of infusion at 1.0 μl/min, the treatment volume obtained using the small 30G cannula (outer diameter, d = 0.31 mm) was about twice that obtained using the 22G cannula (outer diameter, d = 0.72 mm). After infusion was stopped, the treatment volumes acquired through diffusion increased at about the same rate regardless of what cannula had been used.
DISCUSSION AND CONCLUSION
Diffusion
As seen in the images from the diffusion experiment and in past literature, dye will diffuse outward from the point of delivery over time causing flatter concentration profiles (Nicholson 1985). Longer time periods allow the dye to penetrate a larger area of the agarose gel. The diffusion experiments were preformed to examine the difference in the distance covered from simple diffusion to CED. Although CED is necessary to by pass the blood brain barrier, diffusion has been used in the past for smaller molecules, thus, it is a reasonable starting point. Furthermore, these diffusion experiments were the benchmarks used to determine species diffusivity. CED is more advantageous than diffusion due to the ability to achieve controlled target area in contrast to diffusion.
Convection-Enhancement
When a cannula is placed in the agarose before setting, reflux is minimized, as confirmed by the results, because the gel is not injured. Low flow rates and smaller diameter cannulas minimize reflux and produce round, controlled infusion patterns. The infusion patterns seen in these trials are optimal for targeting a specific area of the brain for drug delivery in patient studies. For CED, it was necessary to explore different diameters in order to determine its influence on area of infusion and to test whether cannula size would change the infusion pattern.
Quantitative results as shown in Figure 7 have illustrated the fact that convection-enhanced delivery produces larger treatment volumes than diffusion alone. Because of bulk flow during CED, the high relative dye concentration at the infusion site is effectively carried radially outward, with diffusion playing but a small role in shaping the concentration profile. The result is a nearly trapezoidal concentration profile with a very narrow band of intermediate concentrations between high and low. This type of concentration profile would be desirable in the clinical setting, because the targeted area of the brain would receive a high therapeutic dose while surrounding tissues would receive only a very low dose. After cessation of CED, only diffusion is responsible for the transport of dye molecules. This causes the dye concentration to gradually decrease at the center of the profile, while the slopes decrease in steepness. A much flatter concentration profile results, with a wide band of intermediate concentrations.
The occurrence of reflux, or leak-back along the pathline of the cannula, played a significant role in the preliminary stages of infusion testing. Reflux is a major issue in drug delivery because of the toxicity of infusate to untargeted areas. In reflux, an unspecified amount of infusate spreads radially outward from the infusion site results thus limiting the achievable treatment volume. Cannula size and infusion rate have been shown to affect the extent to which reflux occurs, but preinfusion sealing time has been mentioned as a possible means to eliminate reflux. Without knowing the exact volume of dye that leaks back along the cannula, analysis on this phenomenon is difficult.
The resulting concentration field developed due to the CED infusion displays the fact that convection plays an important role in creating and sustaining a high concentration profile which is needed in various forms of drug delivery treatments. In the larger scope of trials, the effects of CED on tissues must be considered in order to effectively create a protocol for infusive treatments. Long range applications will rely on the positive outcome of the in vivo trials. A complete biological protocol for drug delivery into the human brain is unavailable at this time due to the lack of comparison in tissues and analysis of the phenomenon of reflux. Improvements in drug delivery methods will require extensive input and thorough analysis but will profess a great impact on the treatment of various neurodegenerative diseases.
ACKNOWLEDGEMENTS
We thank Dr. Andreas Linninger, Mr. Mahadevabharath Somayaji, Dr. Richard Penn, and Dr. David Wright for their help with this project, which was funded by an NSF REU supplement through Dr. Linninger's laboratory.
REFERENCES
Chen M.Y., Lonser R.R., Morrison P.F., et al (1999) Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg, 90: 315-320.
Chen, Z.J., Gillies, G.T., Broaddus, W.C., Prabhu, S.S., Fillmore, H., Mitchell, R.M., Corwin, F.D., Fatouros, P.P. (2004) A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg, 101(2): 314-322.
Chen, Z.J., Broaddus, W.C., Viswanathan, R.R., Raghavan, R., Gillies, G.T. (2002) Intraparenchymal drug delivery via positive-pressure infusion: experimental and modeling studies of poroelasticity in brain phantomgels. IEEE Transactions on Biomedical Engineering, 49(2): 85-96.
Krauze M.T., Saito R., Noble C., et al (2005) Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg, 103: 923-929.
Lieberman D.M., Laske D.W., Morrison P.F., et al (1995) Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg, 82: 1021-1029.
Linninger A.A, Somayaji, M.R, Mekarski, M; Zhang, L. (2008) Prediction of convection-enhanced drug delivery to the Human Brain, Journal of Theoretical Biology, 250: 125-138.
Morrison, P.F., Laske, D.W., Bobo, H., Oldfield, E.H., Dedrick, R.L. (1994) High-flow microinfusion: tissue penetration and pharmacodynamics. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 266(1): R292-R305.
Morrison P.F., Chen M.Y., Chadwick R.S., et al (1999) Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol, 277: R1218-R1229.
Nicholson C. (1985) Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Research, 333: 325-329.
Nicholson, C. (2001) Diffusion and related transport mechanisms in the brain tissue. Reports on progress in Physics, 64: 815-884.
Pardridge, W.M. (1999) Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport systems. Pharm. Sci. Technol. Today, 2(2): 49-59.
Pardridge., W.M. (2005) The blood-brain barrier: Bottleneck in brain drug development. The Journal of the American Chemical Society for Experimental and Neurotherapeutics, 2: 3-14.
Raghavan, R., Brady, M.L., Rodriguez-Ponce, M.I., Hartlep, A., Pedían, C., Sampson, J.H. (2006) Convection enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurgical Focus, 20: 1-13.
Saltzman, W.M., Radomsky, M.L.R. (1991) Drugs released from polymers: diffusion and elimination in the brain tissue. Chemical Engineering Science, 46 (10): 2429-2444.
Somayaji, M.R, Xenos, M, Zhang, L, Mekarski, M, and Linninger A.A. (2008) Systematic Design of Drug Delivery Therapies. Computers and Chemical Engineering, 32: 89-98.
Thorne, R.G., Frey II, W.H. (2001) Delivery of neurotrophic factors to the central nervous system. Clin. Pharmacokinet, 40(12): 907-946.