Author: Mahdieh Khosroheidari
Institution: 1Molecular Genetics Lab, Special Medical Center, Tehran, Iran
Date: August 2008
ABSTRACT
Cocculus cordifolius, a climbing shrub found in tropical Western India, Burma and Ceylon, whose parts are known to be used as traditional herbal medicine to treat various aliments such as cold fevers, seminal weakness and urinary affections, stomach and splenic affections, and chronic gonorrhea. The solvent fractions of the whole plant of C. cordifolius were extracted with hexane, dichloromethane, ethyl acetate and methanol to determine their antimicrobial and anti-inflammatory activities. The methanol and ethyl acetate extracts showed a significant inhibition on the growth of Gram (+) and Gram (-) bacteria besides Candida albicans at concentrations of 500 µg/ml and 1000 µg/ml. Methanol and ethyl acetate extracts exhibited good anti-microbial activities against all human pathogens tested except Klebsiella pneumoniae. Cyclooxygenase (COX) or prostaglandin endoperoxide H synthase (PGHS) enzymes are used widely to measure the anti-inflammatory effects of natural products and are the pharmacological target site for non-steroidal anti-inflammatory drug (NSAID) discovery. IC[SUB]50[/SUB] of COX-1 by methanol and dichloromethane extracts were 12 µg/ml and 12.5 µg/ml and that of IC[SUB]50[/SUB] of COX-2 were 14.5 µg/ml and 15.5 µg/ml respectively. Methanol and dichloromethane extracts showed significant and dose dependent inhibition with Bacillus subtilis spore suspension, COX-1 and COX-2.
INTRODUCTION
Many of the drugs isolated and characterized from plants and extensively used in modern medicine have a folklore origin and are traditionally employed in systems of medicine in curing many aliments (Dobner et al., 2003; Baydar et al., 2004; Chandrasekaran et al., 2004). Several drugs and chemotherapeutics have been obtained from naturally occurring products of medicinal plants (Chattopadhyay et al., 2001; Chattopadhyay et al., 2002).
Cocculus cordifolius, is a common climbing shrub found growing on other high trees in tropical Western India (grows wild on hedges at Ahmadabad), Burma and Ceylon. The parts of the plant are known to be used as traditional herbal medicine to treat various aliments such as cold fevers, seminal weakness and urinary affections, stomach and splenic affections, and chronic gonorrhoea in various parts of India, Burma and Ceylon. The stem is bitter, aphrodisiac, hepatic stimulant, antiperiodic, mild diuretic and demulent. Parts of stem are largely used as a valuable nutrient, treatments of seminal weakness, minor infections and general debility.
Nonsteroidal anti-inflammatory drugs's, anti-inflammatory action, is due to the reduced synthesis of bioactive prostanoids which is brought in by the inhibition of cyclo-oxygenase (COX). COX 1 is constitutively produced in almost all tissue cells and is involved in the synthesis of prostaglandins (PGs) for regulation of normal cellular processes (Touhey et al., 2002). COX 2, on the other hand, is highly inducible in response to proinflammatory stimuli, cytokines and mitogens resulting in exaggerated PG release. Many scientists have reported a proinflammatory role for COX-2, suggesting that its inhibition would be a major target for the future treatment of inflammatory arthropathies and that cyclooxygenases (COX) produce various types of prostaglandins, which are implicated in various physiological events including progression of inflammation, imunomodulation, and transmission of pain.
Figure 1. Concentration dependent inhibition of germination of B. subtilis spores by solvent extract fractions of C. cordifolius. The presumptive screening of anti-proliferative activities was determined using inhibition of B. subtilis spores in seeded agar medium. The 50% inhibition of spore germination was observed at 4.3 and 4.5 mg/ml concentration of methanol and dichloromethane extracts respectively.
In this work we herein report the results of antimicrobial activities of the solvent extract fractions on a wide variety of Gram positive and Gram negative medically important bacteria. Methanol and ethyl acetate fractions are effective in inhibiting the growth of C. albicans and Gram (+) and Gram (-) human pathogens excepting K. pneumoniae. The anti-proliferative bioassay on B. subtilis spore suspension revealed that methanol and dichloromethane fractions effectively inhibited in a dose dependent manner. The fractions also inhibited cyclooxygenases (COX-1 & COX-2) which are predominately used as markers in anti-inflammatory studies.
MATERIALS AND METHODS
Plant material
Cocculus cordifolius plants collected in 2003 from Angaram, Marakanam, a local regional area between Pondicherry and Chennai in TamilNadu, India, were identified by the Department of Botany, Pondicherry, India and confirmed with Materia Medica, [1969] (Table 1).
Extraction
The finely ground powdered of the air-dried plant was extracted sequentially from non-polar to polar solvents namely hexane, dichloromethane, ethyl acetate, and methanol. After each step, the extracts were filtered and the filtrate was concentrated by rota-evaporator.
Antimicrobial activity
Antimicrobial activities were performed on different Gram (+), Gram (-) bacteria and fungi. Antibacterial properties were determined by agar diffusion method as described by Chandrasekaran et al., 2004. For the antibacterial assay, the following microorganisms were employed - Gram (+) bacteria - Bacillus subtilis, Micrococcus, Staphylococcus aureus and Gram (-) bacteria - Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Salmonella typhi and Shigella flexneri. Antifungal properties were evaluated as described by Chandrasekaran et al., 2004, using Candida albicans. Each extract was dissolved in 5% dimethyl sulphoxide (DMSO) at concentrations of 500 and 1000 μg/ml and impregnated and dried onto 6 mm diameter Whatman no. 1 sterile filter paper discs was aseptically added over. In vitro antibacterial activity was determined by using Mueller Hinton agar and In vitro antifungal activity was determined by using Sabouraud Dextrose agar. The zone of inhibition was measured from the edge of the disc to the inner margin of the surrounding pathogens. Each assay in this experiment was repeated twice.
Presumptive anti-proliferative bioassay with Bacillus subtilis (Catino et al., 1985; Nadyne et al., 2007)
B. subtilis was plated on a Brain heart infusion agar medium and incubated at 37C for 48h and subsequently at room temperature (R/T) for 20 days. 100 ml of sterilized water was added to the culture to make a spore suspension. The suspension was heated at 55-60C for 30 min and stored in a refrigerator. 1 ml of the spore suspension of B. subtilis was mixed with 100 ml of Davies synthetic medium at 42-43C and the mixture was immediately plated on a Petri dish. The test samples incorporated in discs at 5 µg/ml were placed on the agar surface. After incubation at 37C for 24 h, the zone of inhibition was measured.
Cyclooxygenase inhibitory assay
The ability of each solvent extract to inhibit COX-1 and COX-2 (IC[SUB]50[/SUB] value, µg/ml) was determined using Colorimetric COX Inhibitor Screening Assay kit (catalog number 760111, Cayman Chemical, Ann Arbor, MI, USA). Different concentrations of each of the extracts in DMSO were employed (5, 10, 20, 40, 60, 80 and 100µg/ml) to determine the IC[SUB]50[/SUB]. The procedure was carried out as per the manufacturer's guidelines laid out in the COX Inhibitory assay kit provided by Caymen Chemicals, USA. DMSO was used as solvent control. SC-560, a positive control provided along with the kit was used as standard.
RESULTS
Antimicrobial activity
Antimicrobial activity of solvent extracts fractions from C. cordifolius were tested against a few important human pathogenic bacteria and C. albicans (fungi) by agar diffusion method and the results are shown in Table 2. The methanol and ethyl acetate extracts of C. cordifolius at the concentration of 500 µg/ml and 1000 µg/ml significantly inhibited the growth of almost all the Gram (+) and Gram (-) bacteria besides C. albicans tested except Klebsiella pneumoniae.
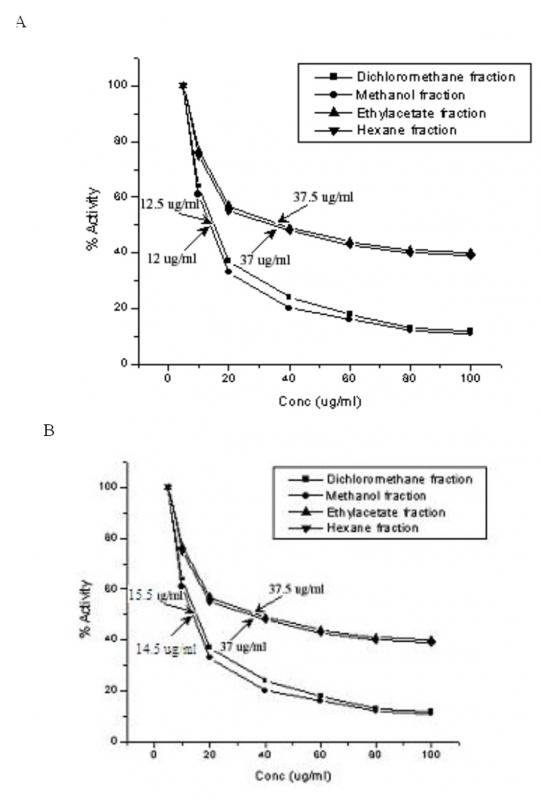
50[/SUB]. The IC[SUB]50[/SUB] of COX-1 by methanol and dichloromethane extracts were 12 µg/ml and 12.5 µg/ml and that of IC[SUB]50[/SUB] of COX-2 by methanol and dichloromethane extracts were 14.5 µg/ml and 15.5 µg/ml respectively.']
Figure 2. Influence of solvent extract fractions of C. cordifolius on (A) COX-1 and (B) COX-2 activities. The values shown against the arrows are IC[SUB
Presumptive anti-proliferative bioassay
To determine whether the extracts did show any anti-proliferative activity, a more conventional, crude method was employed using B. subtilis spores suspension along with the extracts at varying concentrations and the percent inhibition was calculated. The results clearly indicated that the inhibition of spore germination is concentration dependent and 100% inhibition was seen at 100 mg/ml concentration (Fig. 1). The 50% inhibition of spore germination was observed at 4.3 and 4.5 mg/ml concentration of methanol and dichloromethane extracts respectively.
Cyclooxygenase enzymes (COX-I and COX-II) Inhibitory Assay
Cyclooxygenase (COX) or prostaglandin endoperoxide H synthase (PGHS) enzymes are used widely to measure the anti-inflammatory effects of natural products and are the pharmacological target site for non-steroidal anti-inflammatory drug (NSAID) discovery (Cryer and Feldman, 1998). IC[SUB]50[/SUB]s for inhibition of COX-1 and COX-2 by all the 4 extracts are shown in Fig. 2. The IC[SUB]50[/SUB] of COX-1 by methanol and dichloromethane extracts were 12 µg/ml and 12.5 µg/ml and that of IC[SUB]50[/SUB] of COX-2 were 14.5 µg/ml and 15.5 µg/ml respectively.
DISCUSSION
Antimicrobial activity of C. cordifolius
Many workers have reported on the anti-bacterial and anti-fungal activities of natural products obtained from various sources of plant materials such as seeds (Chandrasekaran et al., 2004), roots (Dobner et al., 2003), plant parts (Baydar et al., 2004) etc. Great attention is directed towards isolation, identification and synthesis of the natural products active against a wide variety of bacteria and fungi that cause diseases both in humans and animals. In this direction, antimicrobial activity of extracts from C. cordifolius was tested against a few important human pathogenic bacteria and C. albicans (fungi) and the results are reveal that methanol and ethylacetate extracts of C. cordifolius at the concentration of 500 µg/ml and 1000 µg/ml significantly inhibited the growth of Gram (+) and Gram (-) bacteria besides C. albicans than the other solvent extracts. It is not known as to why invariably all the extracts did show lesser inhibition against Klebsiella pneumoniae. As suggested by (Chandrasekaran et al., 2004) this could be due to the permeability of the complexes or resistance mechanism displayed by K. pneumoniae against these extracts. From our studies it clearly emerges out that only the methanol and ethylacetate extracts possess anti-microbial activity.
Presumptive anti-proliferative effect of the extracts
To determine whether the extracts did show any anti-proliferative activity, a more conventional method was employed. The inhibition of germination of B. subtilis spores in the presence of added extracts would mean presumable inhibition of cell proliferation. According to this rationale the hexane, dichloromethane, ethyl acetate and methanol extracts were incubated with B. subtilis spores at varying concentrations and the percent inhibition was calculated. The 50% inhibition of spore germination was observed at 4.3 and 4.5 mg/ml concentration of methanol and dichloromethane extracts respectively. Since the cell wall composition of B. subtilis resembles that of the cell wall composition of cancerous cells, many scientists (White, 1982; Catino et al., 1985; Nadyne et al., 2007) use this method to test for presumptive anti-cancer properties.
Cyclooxygenase enzymes (COX-I and COX-II) Inhibitory Assay
Cyclooxygenase (COX) or prostaglandin endoperoxide H synthase (PGHS) enzymes are used widely to measure the anti-inflammatory effects of natural products and are the pharmacological target site for non-steroidal anti-inflammatory drug (NSAID) discovery (Cryer and Feldman, 1998). The formation of prostaglandins from arachidonic acid by prostaglandin synthase is a well-studied process and the effects of the prostaglandins can result in the stimulation of inflammation and associated pain (Cryer and Feldman, 1998, Touhey et al., 2002). Two isozymes involved in the conversion of arachidonic acid to prostaglandins are COX-1 and COX-2 (Cryer and Feldman, 1998, Touhey et al., 2002). Rankings of the extracts reflect their relative selectivity to COX-1 or COX-2 (data not shown). According to Cryer and Feldman (1998), a ratio of COX-2 IC[SUB]50[/SUB] to COX-1 IC[SUB]50[/SUB] close to 1 indicates nearly equal selectivity. Therefore, ratios of >1 indicate that a drug is more COX-1 selective and vice-versa. Based on this logic methanol and dichloromethane extracts qualify to be more selective in inhibiting COX-2 activity. Employing indomethacin and their analogs, Touhey et al., (2002) have reported varying inhibitions of COX-1 and COX-2 activities suggesting that removing chlorine from the benzene ring or changing its position from para-, or replacing it with another halogen, affected the ability of the compound to inhibit COX-2. To the best of our knowledge this is the first report wherein the inhibition of COX-1 and COX-2 activities by C. cordifolius are shown. From our studies the methanol and dichloromethane fractions prove to be significantly toxic to cyclooxygenases and hold much promise for their use as effective anti-inflammatory agents upon purification and further study.
In conclusion, the obtained results clearly showed the presence of antimicrobial and anti-inflammatory activities in the methanol extract of C. cordifolius plants supporting the traditional use of this plant in some bacterial infections and inflammatory conditions and confirm the presence of active chemical compounds related to these activities. Further investigations are going on in our laboratory to isolate and characterize the active components of the plant extract.
REFERENCES
Baydar, H., Sagdiç, O., Özkan, G., Karadogan, T., 2004. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control, 15, 169-172
Catino, J.J., Francher, D.M., Edinger, K.J., and Stringfellow, D.A. 1985. A microtitre cytotoxicity assay useful for the discovery of fermentation-derived antitumor agents. Cancer Chemother Pharmacol. 15(3):240-3.
Chandrasekaran, M., Venkatesalu, V., 2004. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharm., 91, 105
Chattopadhyay, D., Arunchalam, G., Mandal, A.B., Sur, T.K., Mandal, S.C., Bhattacharya, S.K., 2002. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharm., 82, 229237.
Chattopadhyay, D., Maiti, K., Kundu, A.P., Bhadra, R., Mandel, S.C., Mandal, A.B., 2001. Antimicrobial activity of Alstonia macrophylla: a folklore of bay islands. J Ethnopharm., 77, 4955.
Cryer, B., Feldman, M., 1998. Cyclooxygenase-1 and Cyclooxygenase-2 Selectivity of Widely Used Nonsteroidal Anti-Inflammatory Drugs. The American Journal of Medicine®, 104, 413-421.
Dobner, M.J., Schwaiger, S., Jenewein, I.H., Stuppner, H., 2003. Antibacterial activity of Leontopodium alpinum (Edelweiss). J Ethnopharm., 89, 301-303.
Nadyne A. Luis, Ping Zhu and Guangyi Wang. 2007. Identification, Phylogenetic Characterization, and Preliminary Bioactivity Screening of Bacteria Isolated from Suberites zeteki, a Hawaiian Sponge. J. Young. Investigators. 16 (2). http://www.jyi.org/research/re.php?id=869
Touhey, S., O'Connor, R., Plunkett, S., Maguire, A., Clynes, M., 2002. Structureactivity relationship of indomethacin analogues for MRP-1, COX-1 and COX-2 inhibition: identification of novel chemotherapeutic drug resistance modulators. European J Cancer, 38, 1661-1670.
White, R.J. 1982. Microbiological Models as Screening Tools for Anticancer Agents: Potentials and Limitations. Annual Review of Microbiology. 36: 415-433.