Author: Johnathan Zandrew Cheng
Institution: University of Hawaii
Date: March 2007
Abstract
Bacterial biodegradation is the basis for the bioremediation of contaminated sites. The key to its success is the use of bacterial strains with high metabolic activity for target contaminants. The goal of this project is to understand the bacterial biodegradation of dinitrotoluene (DNT) by a novel marine bacterium, Pseudoxanthomonas sp. JA40. Isolated along with forty other bacterial species from contaminated Johnston Atoll sand in 2003, JA40 is hypothesized to degrade DNT because it evolved in an environment deficient of organic nitrogen sources but rich in polycyclic aromatic hydrocarbons (PAH's) and polychlorinated biphenyls (PCB's). The new JA40 species has also been identified as a facultative anaerobe making it well suited for in-situ remediation of explosive contaminated coastal areas. The results of this study revealed that JA40 growth and DNT degradation are optimized in anaerobic conditions. The identification of metabolites present in both aerobic and anaerobic JA40 cultures, using GC-MS analysis, suggests the bacterium's ability to consume DNT as both a nitrogen and carbon source.
Introduction
The field of bioremediation research uses biological agents to degrade and neutralize hazardous environmental pollutants. In the 1960's the earliest bioremediation applications involved the treatment of sewage using biofilms on rock filters (World Water Council, 2005). By the 1970's studies had evolved into the use of microbes in the bioremediation of oil in water (Kator, 1973; Shmaefsky, 1999). Since then, bioremediation research has expanded significantly to introduce novel techniques such as biostimulation, bioventing, and biofiltration to remove environmental contaminants (Alexander, 1994).
Bioremediation is based on the principle that microorganisms grow on available carbon, nitrogen, and phosphorus, some of which are contained in toxic compounds. Early bioremediation research was dedicated to obvious sources of carbon contaminants such as petroleum materials, while nitrogen and phosphorus sources were provided in abundance (Atlas, 1981; Alexander, 1994; Margesin, 2001). In recent years, however, research has begun to shift towards the bacterial bioremediation of pesticides and munitions, compounds in which the nitrogen source is considered the contaminant. In these studies the carbon and phosphorus sources were provided in sufficient quantity while nitrogen containing s-triazine compounds such as atrazine and dinitrotoluene were targeted (Oh, 1998; Nishino 2000; Neumann et al., 2004). The natural affinity of bacteria toward these basic elements makes it an invaluable tool for controlling the increasing numbers of polluted sites.
The remediation of chemically contaminated sites has become a priority for many organizations with the establishment of the United States Environmental Protection Agency in 1970 (Environmental Protection Agency, 2005). As a result, the field of bioremediation research has matured to the point that it is now accepted as a cost effective way to eliminate chemical pollutants from waste sites (Alexander, 1994; Shmaefsky, 1999; Aken, 2003) and is projected to receive increased investment in the near future (World Water Council, 2005).
The feasibility of a specific bioremediation technology is determined by a variety of parameters, which include biotic and abiotic factors as they relate to the diverse conditions of the natural environment. Biological factors include the rate at which the microorganism breaks down a compound, the availability of target and inhibitory compounds, and the toxicity of the degradation products to the microorganism (Dibble, 1979; Neumann et al., 2004). Abiotic factors include the target compound's volatility and likelihood of photodegradation (Alexander, 1994; Eweis et al., 1998). It is also a fundamental issue that the costs associated with using these bioremediation methods are less than the cost of the current technologies employed. Nevertheless, researchers must face the challenge of replicating and optimizing natural degradation processes, before they can be certain about employing a new bioremediation method as a suitable solution (Alexander, 1994).
The removal of DNT from contaminated areas has been of primary concern due to its potential carcinogenic effects toward humans. Symptoms of DNT poisoning include headache, methemoglobinemia, and cyanosis (Christopher, 2000; Environmental Protection Agency, 1986). DNT has been extensively found in the soil and groundwater of previous TNT manufacturing sites, leading to the contamination of waste treatment systems (Nishino et al., 2000). The bioremediation of dinitrotoluene has been previously studied with microorganisms (Oh et al., 1998; Nishino et al., 2000). The complete mineralization of DNT as a carbon source was accomplished by the bacteria Psuedomonas sp. (Spanngord, 1991). Recently, dinitrotoluene was successfully utilized as a primary nitrogen source for the bacterial strain Burkholderia cepacia R34 (Johnson, 2002). However, the DNT degrading bacteria strain was only able to degrade 2,4-DNT to the 2,4-dihydroxy-5-methyl-6-oxo-2,4-hexadienoic acid (Johnson, 2002). Even though noticeable amounts of DNT were degraded, Burkholderia cepacia R34 was not able to achieve complete mineralization of DNT. Due to the few known bacteria capable of degrading DNT, the bioremediation of explosives remains an area of continuing research interest.
This work presents new information on the capability of a novel marine bacterial isolate, Pseudoxanthomonas sp. JA40 (Harada et al., submitted), to degrade DNT in the presence of high salinity. Isolated along with forty other bacterial species from contaminated Johnston Atoll sand in 2003; JA40 is hypothesized to degrade DNT containing explosives because it evolved in an environment deficient of organic nitrogen sources but rich in polycyclic aromatic hydrocarbons (PAH's) and polychlorinated biphenyls (PCB's). Outcomes of this work may contribute to the limited body of knowledge that currently exists on bacteria capable of degrading explosives (Esteve-Núñez, 2001). JA40's tolerance to high salinity also bodes well for its potential use in coastal zone management.
Materials and Methods
Cell Line Maintenance:
The pure JA40 strain was isolated from successive dilutions of PAH and PCB contaminated Johnston Atoll soil spread-plated on Difco Marine Agar (Franklin Lakes, NJ) (Harada et al., submitted). Colonies appearing after 3-5 days of incubation at 30 οC were re-streaked for isolation on the marine agar. Working JA40 cultures were plated onto marine agar and maintained at 30 οC in an incubator. Stock JA40 cultures were kept in Difco Marine Broth (Franklin Lakes, NJ) at -80 οC with 50% glycerol (v/v).
Table 1. DNT Degradation Treatment and Controls at Aerobic Conditions
Basal Salt Medium:
Liquid basal salt medium consisted of 0.875 g K2HPO4, 0.3 g NaH2PO4H2O, 0.125 g MgSO4, 1 mL of trace metal solution, and 5 g NaCl, dissolved in 500 mL of deionized water (Oh, 1998). The added trace metal solution was composed of 0.05 g Na2MoO4H2O, 0.09 g Na2B4O710H2O, 0.08 g FeCl36H2O, 0.065 g CoCl26H2O, 0.085 g CdSO48H2O, 0.08 g CuSO45H2O, 0.05 g ZnSO4, 0.06 g MnSO4H2O per 1000 mL of distilled water. The pH of the basal salt medium was adjusted to 7.20 with 0.1 M sodium hydroxide solution. The medium was sterilized by autoclaving at 120 οC for 20 min. 100 μL of filter sterilized vitamin solution composed of 0.002 g biotin, 0.002 g folic acid, 0.005 g thiamine HCl, 0.005 g vitamin B12, 0.020 g niacin, 0.020 g para-aminobenzoic acid per 100 mL of distilled water, was added after autoclaving to prevent vitamin decomposition.
Preparation of JA40 Inoculum:
JA40 colonies were picked from the stock marine agar plates (Franklin Lakes, NJ) and then resuspended in a centrifuge tube containing 1.5 mL of 1% filter sterilized saline solution. After 2 min the bacterial cells were pelleted in a centrifuge for 3 min at 5,000 rpm. The pelleted cells were then washed once and resuspended in 1.5 mL of saline solution prior to use as an inoculum for either aerobic or anaerobic growth.
Aerobic Growth of JA40:
650 μL of the JA40 innoculum, 250 μL of a 0.35 g/ml glucose solution, and 100 μL of a 125,000 ppm DNT solution (in acetone) were added into Erlenmeyer flasks containing 250 mL of basal salt medium (Table 1). Specifically, three controls, one without glucose, one with no bacteria, and one without DNT were set up. The flasks were capped with a 0.45 micron Whatman Bug and placed inside an incubator shaker held at 180 rpm for a total of 29 days at 40 οC.
Anaerobic Growth of JA40:
500 μL of the JA40 innoculum, 15 μL of 0.35 g/mL glucose solution, and 6 μL of 125,000 ppm DNT solution (in acetone) were added into appropriate test tubes containing 15 mL of basal salt medium (Table 2). Similar to the aerobic experiments, 3 sets of controls were used as defined in Table 2. Each test tube was purged with grade 5.5 nitrogen for a total of 3 min before placement in an incubator set at 40 οC for a total of 36 days.
Table 2. DNT Degradation Treatment and Controls at Anaerobic Conditions
Sampling:
Optical density readings for aerobic growth of JA40 were collected on days 15, 22, and 29. Readings for the anaerobic growth were measured on days 22 and 36. All absorbance readings were taken at 600 nm wavelength using Varian Gary 50 Bio UV-visible spectrophotometer.
An aliquot of 25 mL aerobic culture supernatants was taken on days 14, 21, and 28 for DNT and metabolite extraction. An aliquot of 15 mL anaerobic culture supernatants was taken on days 22 and 36 for DNT and metabolite extraction.
Extraction of DNT and Metabolites from Aerobic and Anaerobic Basal Salt Medium Cultures:
The extraction of DNT and its metabolites from aerobic basal salts media culture was executed using liquid-liquid extraction of 25 mL samples of culture supernatant against 12.5 ml of ethyl acetate. Three consecutive extractions were conducted with the ethyl acetate layer from each separation collected and filtered through a glass funnel containing sodium sulfate. The ethyl acetate extract was then concentrated to dryness using a rotary evaporator. The DNT containing residue was then redissolved in 5 mL of a 1:1 mixture of methanol:acetonitrile and a 1 mL aliquot was sampled for LC-MS analysis (Campbell et al., 2003). A separate 2 mL aliquot of the redissolved DNT residue was concentrated to 0.2 mL using a nitrogen evaporator for subsequent GC-MS analysis.
The extraction procedure of DNT and metabolites from anaerobic basal salt medium culture was the same as that for the aerobic cultures, except with reduced volumes. The total amount of culture was 15 mL extracted three times with 7.5 mL of ethyl acetate. The DNT containing residue was redissolved in 5 mL of a 1:1 mixture of methanol:acetonitrile, where a 1 mL aliquot was sampled for LC-MS analysis and a separate 2 mL aliquot was concentrated to 0.2 mL for GC-MS analysis.
LC-MS Analysis of DNT:
DNT calibration standards were prepared from standard grade 2, 4-dinitrotoluene to 10, 25, 50, 75, and 100 ppm. An Agilent 1100 LC-MS (Wilmington, DE) was used for the analysis. The separation column was a Hewlett Packard Zorbax Column SB-C18 (narrow bore 2.1 cm x 150 mm x 5 μm) (Palo Alto, CA). The column flow rate was held at 0.7 mL/min and the temperature was maintained at 44 °C. During the first 15 min, the solvent carrier was a water-methanol mixture of 75:25 that was linearly reduced to 65:35. In the next 5 minutes the methanol-water mixture was stepped back up to the 75:25 ratio and then held for 5 min. While the standard injection volume was 15 μL, the sample injection volumes were limited to 2 μL in order to adjust for the highly concentrated DNT in the sample extracts. The elution was recorded at 254 nm along with a full UV spectrum (Campbell et al., 2003). Dinitrotoluene was identified and quantified from the resulting chromatogram and mass spectra.
GC-MS Analysis of DNT Metabolites:
DNT metabolites were identified using a Varian 3800 GC and a 1200 Quadrupole MS (Walnut Creek, CA). A Varian Factor Four Column (30 m x 0.25 mm x 0.25 μm) VF-5ms (Walnut Creek, CA) was used. The injector temperature was held at 210 °C, while injection volumes were at 1 μL per sample. The temperature in the GC oven was held at 80 °C for 1 min then increased to 320 °C at a rate of 10 °C per min and held there for 2 min. The helium flow rate was maintained at 1.0 mL/min. Ion detection by the MS was held in the 45 to 550 amu range.
Results
Aerobic JA40 Bacterial Growth
The JA40 bacteria cultured in aerobic conditions showed little to no growth throughout 28 days of incubation, with only minor fluctuations in absorbance readings centered around zero and no change in culture turbidity.
Aerobic JA40 DNT Degradation
As the concentration of DNT on day 28 was equivalent to that in the control (88%), the expected dinitrotoluene degradation was not observed for aerobic growth of JA40 (Table 3).
Table 3. DNT Aerobic Degradation by JA40 bacteria from 25 mL of Culture Media Sampled for Extraction on Days 14, 21, and 28 Percent DNT Remaining with Mean Standard Deviation for n = 3.
Anaerobic JA40 Bacterial Growth
JA40 bacterial cultures showed an increase of approximately 0.3 in absorbance readings from days 22 to 36 (Figure 1B). The growth observed in the JA40 inoculated flasks are substantially larger than that observed for the DNT control. The growth the JA40 containing cultures is inversely related to DNT degradation over days 22 to 36 (Figure 1A). This result is considered accurate as the DNT control showed little growth and DNT degradation. Comparing these numbers with the values of JA40 in aerobic conditions (Table 3), the JA40 in anaerobic conditions had considerable growth and DNT degradation.
Figure 1. Anaerobic JA40 bacteria growth and DNT degradation, sampled on days 22 and 36 for n = 1. A) Percent DNT remaining in cultures composed of JA40+DNT+Glucose (●), DNT+Glucose (■), and JA40+DNT with no carbon source (▲). B) Bacterial growth readings taken at 600 nm for the cultures JA40+DNT+Glucose (●), DNT+Glucose (■), and JA40+DNT with no carbon source (▲).
Anaerobic JA40 DNT Degradation
Dinitrotoluene breakdown was observed in the anaerobic DNT degradation by JA40 (Figure 1A), with DNT levels in the JA40 lowered to 48% by day 36. By contrast, the control showed no such pattern by day 22, suggesting the degradation trend to be valid. Recovery from the DNT control was at 74% on the same day (Table 4). After day 22, as the DNT levels for the JA40 containing flask continued to decrease while the DNT control did not (Figure 1A), a consistent degradation trend developed.
Comparison of the two anaerobic JA40 cultures reveals that greater degradation of DNT occurs in association with the glucose carbon source consumption. This result is clearly indicated on day 36 where the DNT levels remaining in the JA40 cultures in the presence and absence of carbon were 27% and 48%, respectively (Table 4). The DNT degradation rate increased when JA40 was facilitated with a carbon source.
Table 4. DNT Anaerobic Degradation by JA40 bacteria from 15 mL of Culture Media Sampled for Extraction on Days 22 and 36 Percent DNT Remaining for n = 1. * DNT Levels Undetectable During LC-MS Analysis on Day 22
DNT Metabolites
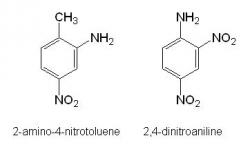
Dinitrotoluene metabolites were found in both aerobic and anaerobic JA40 culture extracts deficient of an added carbon source. The metabolites included 2-amino-4-nitrotoluene, an expected product from the reduction of a nitro group to an amine group on dinitrotoluene (Figure 2). Along with this metabolite, the 2, 4-dinitroaniline compound was also observed (Figure 2) suggesting that JA40 may have acquired a carbon source by converting the methyl group of dinitrotoluene into an amine group. No such metabolites were identified in the aerobic and anaerobic JA40 cultures containing an added carbon source. Furthermore, no metabolites could be identified in both aerobic and anaerobic JA40 cultures towards the last sampling date.
Figure 2. Structures of the DNT metabolites 2-amino-4-nitrotoluene and 2, 4-dinitroaniline identified in aerobic and anaerobic culture media containing JA40.
Discussion
Aerobic JA40 Growth and DNT Degradation
The absence of significant bacterial growth in the aerobic cultures verifies JA40's slow overall growth rate (Harada et al., submitted). Comparing the incubation periods of the JA40 aerobic cultures to the anaerobic cultures, a longer incubation period may have been required for significant bacterial growth to be observed. The absorbance values taken for the first 28 days of incubation may have just been a record of an extended lag phase for the slow growing JA40 bacteria. If the incubation time were increased to 36 days, as in the anaerobic cultures, a greater JA40 aerobic growth might be achieved.
Even though the lack of DNT degradation remains consistent with the absence of bacterial growth in the aerobic cultures (Figure 1B), there still remains a possibility that JA40 can degrade DNT if a longer incubation period was allowed for the bacterium to grow. As in previous research conducted on the bacterial degradation of DNT, levels of dinitrotoluene always remained at preliminary levels until bacterial growth was observed (Nishino, 2005). Therefore, it can only be concluded that DNT degradation is not observed during long periods of lag phase, and that aerobic environments may not be the best place to promote DNT degradation.
Given the observed bacterial growth under anaerobic conditions, it is likely that JA40 can breakdown DNT in aerobic conditions. The reduced degradation may be due to the presence of a constant oxygen supply in aerobic cultures, perhaps decreasing the immediate need for the JA40 bacterium to reduce the nitro groups for respiration (Grishchenkov, 2000). If true, this would explain the slower rate of DNT degradation observed for aerobic growth. In order to test this idea, JA40 can be grown in aerobic conditions and tested for the degradation of amino-nitrotoluenes. If greater degradation does result, over the same time interval, then this test may imply that the reductions of the nitro groups are the limiting step in the degradation pathway of DNT.
Anaerobic JA40 Growth and DNT Degradation
The absorbance values for days 22 and 36 for JA40 anaerobic growth follow the pattern of exponential bacterial growth that is expected (Spanggord 1991; Oh, 1998; Nishino 2000). The decrease in DNT levels verifies the growth of JA40 in anaerobic culture (Figure 1A), as previous studies on bacterial biodegradation have indicated similar trends (Oh, 1998; Nishino 2000).
The inverse relation that is observed between the increase in bacterial growth and the decrease in DNT remaining in the anaerobic JA40 cultures (Figure 1) suggests that the bacteria are growing due to their consumption of DNT. These similar observations were made in past studies of dinitrotoluene degradation, while comparing bacterial growth to remaining levels of DNT over a timeline (Oh, 1998; Nishino, 2005). These results suggest that JA40 is capable of using DNT in anaerobic conditions. Having made the observation that JA40 degrades DNT in oxygen deficient environments, an increased rate of sampling before day 22 and after day 36 would achieve a greater characterization of JA40's functionality in anaerobic conditions.
The finding that DNT degradation by JA40 was faster in the presence of carbon is consistent with previous studies regarding bacterial degradation (Oh, 1998). Bacteria facilitated with a carbon source were shown to degrade target compounds faster. However, the degradation observed in the carbon deficient JA40 culture (Figure 1A) also suggests that the bacterium is able to acquire carbon in addition to nitrogen from DNT. However, further verification of this idea would require the accurate identification and quantification of resulting metabolites. In general, the difference in degradation rates observed in the presence and absence of carbon in the bacterial cultures shows that the degradation of DNT by JA40 is improved with the availability of a carbon source.
DNT Metabolites
In past studies the most common metabolites of DNT such as 4-amino-2-nitrotoluene and 2-amino-4-nitrotoluene are expected to form, as the degrading bacteria reduce nitro groups to amino groups (Noguera, 1997). The presence of an aminonitrotoluene metabolite in the aerobic JA40 cultures was not anticipated, as there was no significant indication of bacterial growth or DNT degradation. However, the identification of this metabolite in the aerobic JA40 culture, with no added carbon source, suggests that DNT could be breaking down at a very slow rate.
The detection of DNT metabolites in the anaerobic JA40 cultures was expected, however, only the carbon deficient culture contained the metabolite 2-amino-4-nitrotoluene. This observation is best explained by considering the difference in degradation rates of the anaerobic JA40 supplemented with and without glucose. Earlier studies regarding the biodegradation of DNT showed that amino-nitrotoluene metabolites were degraded in less than one day after their formation (Christopher, 2000). The detection of the amino-nitrotoluene metabolite was shown to be dependent on the overall rate at which DNT is metabolized. Due to the increased rate at which DNT was consumed in the JA40 anaerobic culture supplemented with glucose, chances of detecting of the amino-nitrotoluene metabolite would have been infrequent. The absence of any metabolites on the last date of sampling in all anaerobic JA40 cultures reconfirms the notion that amino-nitrotoluene metabolites are continuously broken down.
The presence of 2, 4-dinitroaniline in both aerobic and anaerobic JA40 cultures deficient of an added carbon source suggests the notion that the methyl group on the DNT molecule is consumed as a carbon source by the bacteria. It is likely that JA40 uses DNT as a carbon source given the bacterial growth and DNT degradation observed in anaerobic conditions. However, in the aerobic conditions, a longer incubation time would be needed to observe substantial JA40 growth and DNT degradation.
Conclusions
The difference between the JA40 growth and DNT degradation observed in the aerobic and anaerobic cultures suggests the idea that the reduction of the nitro group is an initial step in the degradation pathway of dinitrotoluene. These results are supported by previous work that showed amino-nitrotoluene metabolites resulting from the degradation of DNT (Noguera, 1997; Christopher, 2000). This bacterial process of respiration has also been noticed in previous studies of facultative anaerobes in oxygen deficient environments (Grishchenkov, 2000). Thus the presence of oxygen may actually be limiting the rate of DNT degradation by JA40, implying that JA40 growth and DNT degradation are optimized in anaerobic conditions. Based on the identification of the metabolites, 2-amino-4-nitrotoluene and 2,4-dinitroaniline, the JA40 in aerobic and anaerobic conditions are likely to be using DNT as both a nitrogen and carbon source. However, a greater frequency of sampling, longer incubation times, and a quantification of metabolites would be necessary to confirm this new hypothesis.
Acknowledgements
The author gratefully acknowledges support from NSF grant #02-43600, the University of Hawaii Sea Grant College Program, and NRL award # N00173-05-2-C003. In addition, the author would also like to thank Dr. Michael Cooney for his guidance and leadership in the Marine Science Undergraduate Research Fellowship Summer Program.
References
Aken B, Yoon J, and Schnoor J. (2003) Biodegradation of Nitro-Substituted Explosives
2,4,6 Trinitrotoluene, Hexahydro-1,3,5-Trinitro-1,3,5-Triazine, and Octahydro-1,3,5,7-Tetranitro-1,3,5-Tetrazocine by a Phytosymbiotic Methylobacterium sp. Associated with Poplar Tissues (Populus deltoides x nigra DN34). Applied and Environmental Microbiology. 70:508-517.
Alexander M. (1994) Biodegradation and Bioremediation. Academic Press, CA.
Atlas RM. (1981) Microbial degradation of petroleum hydrocarbons: an environmental
perspective. Microbiological Reviews. 45:180209.
Campbell S, Ogoshi R, Uehara G, and Li QX. (2003) Trace Analysis of Explosives in
Soil: Pressurized Fluid Extraction and Gas and Liquid Chromatography-Mass Spectrometry. Journal of Chromatographic Science. 41:284-288.
Christopher H, Boardman G, and Freedman D. Aerobic Biological Treatment of 2,4-
Dinitrotoluene in Munitions Plant Wastewater. (2000) Water Research. 34(5):1595-1603.
Díaz E, Ferrández A, Prieto M, García J. (2001) Biodegradation of Aromatic Compounds
by Escherichia coli. Microbiology and Molecular Biology Reviews. 65(4): 523-569.
Dibble J and Bartha R. (1979) Effect of environmental parameters on the biodegradation
of oil sludge. Applied and Environmental Microbiology. 37(4):729-739.
Duque E, Haidour A, Godoy F, and Ramos J. (1993) Construction of a Pseudomonas
hybrid strain that mineralizes 2,4,6-trinitrotoluene. Journal of Bacteriology. 175(8): 22782283.
Environmental Protection Agency. (2005) National Environmental Policy Act of 1969
(NEPA). USC. 42:4321-4347.
Environmental Protection Agency. (1986) Health and Environmental Effects Profile for
Dinitrotoluene Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office. Cincinnati, OH. ECAO-CIN-P183.
Esteve-Núñez A, Caballero A, and Ramos J. Biological Degradation of 2,4,6-
Trinitrotoluene. (2001) Microbiology and Molecular Biology Reviews. 65(3):
335352.
Eweis J, Ergas S, Chang D, and Schroeder E. (1998) Bioremediation Principles.
McGraw-Hill, MA.
Grishchenkov V, Townsend R, McDonald T, Autenrieth R, Bonner J, and Boronin A.
(2000) Degradation of petroleum hydrocarbons by facultative anaerobic bacteria under aerobic and anaerobic conditions. Process Biochemistry. 35(9): 889-896.
Harada R M, Campbell S, Li QX. (2005) Luridamonas kalamensis gen. nov., sp. nov., a novel γ-Proteobacterium isolated from Johnston Atoll, North Pacific Ocean. International Journal of Systematic and Evolutionary Microbiology. Submitted.
Hawari J, Halasz A, Beaudet S, Paquet L, Ampleman G, and Thiboutot S. (1998)
Biotransformation of 2,4,6-trinitrotoluene with phanerochaete chrysosporium in agitated cultures at ph 4.5. Applied and Environmental Microbiology. 65(7):29772986.
Johnson G, Jain R, Spain JC. (2002) Origins of the 2,4-Dinitrotoluene Pathway.
Journal of Bacteriology. 184(15): 42194232.
Johnson G, Smets B, Spain JC. (2001) Oxidative Transformation of Aminodinitrotoluene
Isomers by Multicomponenet Dioxygenases. Applied and Environmental Microbiology. 67(12):5460-5466.
Kator H. (1973) The Microbial Degradation of Oil Pollutants. Louisiana State
Universtiy, Center for Wetlands Resources.
Lakshminarayan PG, Bouzaher A, Shogren JF. (1996) Atrazine and Water Quality: An
Evaluation of Alternative Policy Options. Journal of Environmental Management. 48:111-126.
Margesin R, and Schiner F. Bioremediation (Natural Attenuation and Biostimulation) of
Diesel-Oil-Contaminated Soil in an Alpine Glacier Skiing Area. (2001) Applied and Enviornmental Microbiology. 67(7):3127-3133.
Neumann G, Teras R, Monson L, Kivisaar M, Schauer F, and Heipieper H. (2004)
Simultaneous Degradation of Atrazine and Phenol by Pseudomonas sp. Strain ADP: Effects of Toxicity and Adaptation. Applied and Environmental Microbiology. 70(4): 19071912.
Nishino S, Paoli G, Spain JC. (2000) Aerobic Degradation of Dinitrotoluenes and
Pathway for Bacterial Degradation of 2,6-Dinitrotoluene. Applied and Environmental Microbiology. 66(5): 2139-2147.
Noguera D, and Freedman D. (1997) Characterization of products from
biotransformation of 2,4-dinitrotoluene by denitrifying enrichment cultures. Water Environment Research. 69(3), 260-268.
Oh KH, Kim YJ. (1998) Degradation of Explosive 2,4,6-Trinitrotoluene by s-Triazine
Degrading Bacterium Isolated from Contaminated Soil. Bulletin of Environmental Contamination and Toxicology. 61:702-708.
Shmaefsky B. (1999) Bioremediation: Panacea or Fad? Access Excellence at the
National Health Museum: SciTalk Bioremediation Background.
Spanggord R, Spain J, Nishino S, Mortelmans K. (1991) Biodegradation of 2,4-
dinitrotoluene by a Pseudomonas sp. Applied and Environmental Microbiology. 57(11): 32003205.
World Water Council. (2005) World Commission on Water for the 21st century
Recommendations on Harnessing the tools of Bioremediation for Strengthening
Water Security. World Water Vision