Author: Jereme Mason(1), Anne Bockarie(1), Christina Czarnecki(1), A Bryant(1), J Miller(1), R Vath Jr(1), D Burton(2), F Lawn(2)
Institution: 1)Philadelphia University, School of Science and Health; 2)The Schuylkill Center for Environmental Education
Date: December 2006
ABSTRACT
Invasive Asian earthworms (Amynthas hilgendorfi and A. agrestis) have transformed forest ecosystems along the eastern seaboard of the United States by decimating the forest leaf litter layer, shifting nutrient cycles, and altering belowground fine root distributions and microbial dynamics. Natural resource managers are looking for management methods that both control invasive earthworm populations and ameliorate their damaging effects on the forest ecosystem. Healthy fungal populations in forest soils are particularly important for the successful establishment and growth of plants and tree seedlings in reforestation efforts. The purpose of this study is to examine the impact on soil microflora of earthworm control treatments in an urban forest restoration project. Soil microflora were analyzed over a 21 month period at Schuylkill Center for Environmental Education, Philadelphia, PA for a control and two effective earthworm control treatments: sulfur pellets (180 g m-2 ) alone or sulfur with oak leaf litter amendments (10 cm depth). Pre and post levels of fifteen replicates of each treatment and a control were analyzed for bacterial, fungal, yeast, enteric group, coliform and non-coliform levels. Active and ratio of fungal to bacterial biomass were analyzed for a sub-sample basis for each of the three treatments. When invasive earthworm biomass was suppressed in the two sulfur treatments, the total fungal abundance increased and the ratio of active fungal: bacterial biomass returned to within healthy levels for deciduous forest ecosystems. Yeast levels dropped significantly, but still remained much higher post treatment compared to non-earthworm dominated forest soils at other sites. Coliform and non-coliform levels did not change significantly and enteric levels increased only in the sulfur + oak treatment. Our findings are significant for stemming the invasion of exotic earthworms in forest ecosystems, but also for speeding the recovery process by ameliorating their negative impact on the decomposer community.
INTRODUCTION
Abundance, biomass, activity and structure of decomposers in the soil foodweb can be used as indicators of ecosystem health because they are responsible for over 80% of total soil metabolism (Brady and Weil 2004; Coleman et al.1992). The decomposer community of fungi and bacteria recycle vital plant nutrients from leaf litter and animal waste. Bacteria convert nitrogen into plant available forms via the process of nitrification. Fungi decompose woody tissue such as cellulose and lignin and stabilize soil aggregates (Brady and Weil 2004). Decomposer communities in deciduous forest soils of poplar, oak and maple are fungal-dominated; compared to agricultural soils which tend to be bacteria-dominated (Ingham et al. 1985). Growth and establishment of forest tree seedlings is directly dependent on the abundance and activity of fungi in the soil. Many tree and mycorrhizae fungal species have a symbiotic relationship. The fungi aid in transporting water and nutrients to the roots. Without sufficient fungi in the soil, trees such as oaks fail to establish and grow due to poor nutrition. One of the first critical steps in forest restoration is to rebuild the soil microbial community by inoculating seedlings with fungal spores, additions of leaves and mulch to increase fungal habitat and use of erosion-control fabric to stabilize the site (Sauer 1998). Fungal inoculation can have impressive results. Cordell (1997) found that trees were able to be harvested for paper manufacturing in a fifteen year rotation on highly disturbed mine spoils with pH values as low as 2.8 when inoculated with fungi.
Recent studies have shown that invasive plant and animal species from Asia can have devastating impacts on the fungal community in forest soils. Stinson et al. (2006) found that tree seedling growth decreased due to antifungal phytochemical compounds released by garlic mustard (Alliaria petiolota), an exotic plant rapidly invading eastern forests. Likewise, invasive earthworms can negatively impact the fungal community in forests by changing nutrient cycling (Scheu and Parkinson 1994; Steinberg et al. 1997), reducing the organic layer (Alban and Berry 1994) and reducing fine-root distributions (Fisk et al. 2004).
Invasive earthworm species from Europe and Asia are rapidly colonizing and transforming forest ecosystems across the United States (James and Hendrix 2004; Bohlen et al. 2004). Along the eastern seaboard, two invasive earthworm species from Asia have been implicated namely, Amynthas hilgendorfi and Amynthas agrestis, which are concentrated in urban areas and are of increasing concern for natural resource professionals interested in restoration of forest sites (Pouyat et al. 1994; Steinberg et al. 1997; Szlávecz et al. 2006). Introduced to the United States via the shipping and horticultural industries from their native habitat in Southeast Asia (Gates 1982), these invasive earthworms destroy the forest floor habitat by consuming large quantities of leaf litter (Alban and Berry 1994; Hale et al. 2005) and rapidly alter the soil microbial community to favor bacteria over fungi (Wardle 2002; Lavelle et al 1999; Groffman et al. 2004). A study in the Canadian Rocky Mountains, conducted by Scheu and Parkinson (1994), demonstrated that within eight weeks of introduction earthworms reduced the fungal content of the soil from an initial 55% to between 30 and 40%, with a corresponding increase in bacterial biomass. The shift from a fungal to a bacterial-dominated system results in a higher nitrification rate which favors replacement of the native forest understory by nitrogen-loving exotic plant species, results in leaching of excess nitrate into streams and waterways and decreases growth and establishment of native tree species (Sauer 1998). Given the severity of the invasion in many urban sites where the top 6 to 8 cm of soil has been completely replaced by earthworm castes, earthworm control methods are needed.
Fungi appear vulnerable to the actions of invasive earthworms in three respects. Firstly, they serve as a major food source for the animals. Most drastically affected in this regard are fast-growing fungal decomposer species such as Mucor, Trichoderma and Fusarium (Visser 1985). Secondly, large earthworm populations result in soil disturbance which both limits fungal access to organic matter as well as repeatedly disrupting fungal hyphae (McLean and Parkinson 1998). Thirdly, in temperate forests earthworms may bury more than 90% of the available annual leaf litter and with it at least 22 species of the fungi using that food source (Knollenberg et al. 1985). Unlike fungi, several species of coliform and non-coliform decomposer bacteria may survive passage through the earthworm intestine and proliferate in the casts of the animals (Kozlovskaya and Zhdannikova 1961). In addition, certain soil bacterial species, most notably Clostridia, Bacillus and Pseudomonas are able to survive in the presence of earthworms due to endospore and/or antimicrobial production (Kozlovskaya and Zhdannikova, 1961; Kristufek et al. 1993)
Unfortunately, little experimental data exist on effective management strategies to ameliorate the impact of invasive exotic earthworms. Two examples include top-dressing with abrasives such as crushed coal slag decreased castings of Lumbricus terrestris on golf courses in Wisconsin (Williamson and Hong 2004) and electroshocking reduced L. terrestris and Aporrectodea tuberculata populations in agricultural plots in Ohio (Blair et al. 1995). However, neither of these treatments are feasible in forested settings which often have mixed topography and limited access to power lines or roads.
Vermicomposting studies with epigeic earthworm species may provide a starting point for developing effective control treatments based on how populations respond to various environmental factors. In forest ecosystems, epigeic species live and feed primarily in the leaf litter compared to endogeic species which live and feed in the upper soil horizons and anecic species which have deep vertical burrows and feed in the leaf litter (Bouchè 1977; Hendrix and Bohlen 2002; Hale et al. 2005). Epigeic species are used in composting because they are "litter transformers" processing large volumes of undecomposed organic material in short time periods, have a high metabolic and reproductive rate as well as a range of reproductive strategies including parthenogenesis (Domínguez 2004; Hendrix and Bohlen 2002; James and Hendrix 2004). Habitat factors that may be manipulated to influence population levels and reproduction of epigeic species include decreasing soil pH to below 4.5 (Edwards and Bohlen 1996) by adding sulfur or decreasing food quality and availability by incorporating high fiber, low nutrient mulches such as sawdust, pine needles or oak leaves (Domínguez 2004). Likewise, epigeic earthworm populations may be limited through surface applications of organic substances that are irritants or slow growth rates. Compounds in this category include diatomaceous earth, wasabi, tobacco or black walnut (Juglans nigra) hulls.
The present microbial study is based on findings from two previous field experiments (Bockarie et al. In Review). After testing 26 different earthworm control treatments over a three year period, we found two treatments significantly reduced earthworm biomass for different time periods compared to a control. A single fall application of 180 g m-2 sulfur significantly reduced total earthworm biomass for eleven months; whereas, a single fall application of 180 g m-2 sulfur plus 10 cm of oak leaf litter reduced total earthworm biomass from 95 ind m-2 pretreatment to 17 ind m-2 at 21 months post treatment. Soil pH levels dropped in both treatments from pretreatment levels of 6.3 to 6.4 to below 4.5 at seven months post treatment and remained at 5.5 after 21 months. The pH of the control ranged from 6 to 6.5 during this same time period.
The purpose of this study was to measure the soil microflora response over time between the control and the two sulfur treatments which were effective at reducing earthworm biomass. An ideal earthworm management treatment would not only be one that reduced biomass over time, but would also initiate amelioration of damage to the soil foodweb, thereby enhancing restoration efforts at the site. Given that earthworm biomass was reduced for an extended period of time by the treatments, we hypothesized that the bacterial levels would decrease because earthworm casts have high bacterial loads. Further, we predicted that the fungal community would respond positively to the sulfur plus oak leaf litter treatment because the sulfur would lower the soil pH to those typical of a deciduous forest ecosystem and the oak leaves would provide needed habitat for fungi as well as stabilize the soil surface. Given the extent of the damage to the site, we questioned whether this combination of amendments would be sufficient to encourage fungal growth and whether the fungal:bacterial ratio would return to healthy forest levels within the time frame of the study.
The implications and impact of this study are significant for natural resource professionals who are seeking effective methods to both combat the growing impact of invasive species in forest habitats and to restore the function of the soil microbial community. Given that restoration of the soil microbial community is so critical to successful restoration. Any treatments which initiated a trend towards a healthy structure and function of the decomposers would be a positive step in combating the degradation wrought by invasive earthworm species.
MATERIALS AND METHODS
Site Description
The study site is within Penn's Native Acre, a fenced 4.05 ha highly disturbed temperate deciduous forest site undergoing restoration located within The Schuylkill Center for Environmental Education (SCEE), Philadelphia, PA (40o03' N 75o15'W). Philadelphia has a thirty year mean maximum temperature in July of 30oC and a thirty year mean precipitation in July of 107 mm (NOAA-CIRES 2005). Mean annual rainfall is 1,044 mm and there are on average 181 days in the growing season (Clemants and Moore 2003). The study site at SCEE is a large basin surrounded by ridges dominated by tulip poplar (Liriodendron tulipfera) with an adjacent sparse understory of green ash (Fraxinus pennsylvanica), white dogwood (Cornus florida), and spicebush (Lindera benzoin). The soil types are Typic Dystrudepts and Typic Hapludults (USDA 1999). These soils typically have 5 cm of organic matter and 10 cm of loam in the upper surface layer of the soil in non-invaded sites (Tompkins et al. 1975).
Years of over-browsing by white-tailed deer (Odocoileus virginianus) combined with the invasion of the site by Japanese stilt-grass (Microstegium vimineum) and Asian earthworms (Amynthas hilgendorfi and agrestis) has resulted in extremely disturbed herbaceous, leaf litter layer and upper soil horizons. Prior to treatment, the top 8 cm of soil had a pH of 6.4 and was composed entirely of worm casts. Worm densities averaged 89 ind m-2 and all leaf litter is consumed each year by early summer leaving the upper mineral soil horizons bare and exposed. The fungal to bacteria ratio of 0.19 is typical of grassland not a forest.
Restoration began at the site in 2000 with establishment of a 1.8 m high plastic mesh deer fence on the perimeter to exclude deer combined with a bow-hunting program targeted at reducing deer numbers from 154 ind km-2 to the recommended 7 ind km-2 (Shissler and Seidel 1989). The thick matt of stilt grass that dominated the herbaceous layer was controlled using a series of pre and post-emergent herbicides (Pendulum 3.3 with 37.4% Pendimethalin as the active ingredient and EC,Assure II with 10.3% Quizalofop as the active ingredient) (Grover and Burton 2002). Native plant diversity has been promoted through planting several hundred trees, shrubs and forbs as well as selected thinning of the tulip poplar canopy to create light gaps for natural regeneration.
Blocks and Treatments
article_866_order_2
In mid-September 2003, a randomized complete block experiment was established with five blocks of three treatments in 3 m2 treatment plots with a 3 m2 buffer between each plot in the restoration site. The five blocks were selected as they varied in location, degree of erosion, sun exposure and moisture level (Table 1).
Descriptions of the three treatments are shown in Table 2.
article_866_order_3
Sulfur pellets were spread uniformly throughout the treatment plot by hand. Oak leaf litter was collected in a nearby mature stand of oaks and then spread by hand to a uniform depth of 10 cm throughout the treatment plot.
Sampling
Soil samples for total microbial analysis were taken from the three treatments plots in all five blocks prior to treatment (Sept 2003) and 21 months post treatment (May 2005). Soil was collected within two 1 m2 sub-sampling locations which were at opposite corners within each treatment plot. Within each sub-sampling plot, soil was taken from five locations (each of the four corners and the center of the plot) using a 2.5 cm diameter soil core at a depth of 7.5 cm. All ten samples were homogenized for analysis. Samples were placed in individual sandwich storage bags (Ziploc®, S.C. Johnson, Racine, WI), refrigerated and processed for soil microflora in the laboratory within 72 hours.
Laboratory Analysis
The microbial content of the soils was determined using standard methodology which included plating 100 µl of 10-6 suspensions in sterile saline, using nutrient, Sabouraud-dextrose MacConkey and Eosin Methylene Blue (EMB) agars (Tortora 1998). Total counts of all non-fastidious bacteria and mycetes were determined from the nutrient agar plates, with colonial morphology and microscopic appearance following staining being used to differentiate between bacteria, yeasts and molds. Sabouraud-dextrose agar was used to determine total fungal numbers and the remaining agars were differential for environmental coliforms and non-coliforms. All plates were incubated at 30ºC. The number of colonies appearing after 48 hours was determined by direct visual assessment. Each soil sample was replicated a minimum of three times to determine consistent bacterial and fungal numbers.
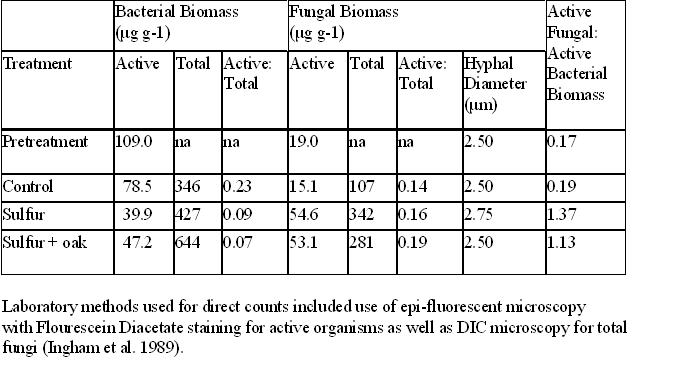
Bacterial and fungal biomass analysis is very expensive at a cost of $100 per sample. Therefore, soil samples for all three treatments from only one block were sent for analysis. These sub-samples were shipped by overnight mail to the Soil Foodweb Inc. Port Jefferson Station, NY. Calculations of active, total and ratio of active to total biomass for fungal and bacterial biomass as well as the ratio of total fungal to bacterial biomass and the fungal hyphal diameter were done. Laboratory methods used for direct counts included use of epi-fluorescent microscopy with Flourescein Diacetate staining for active organisms as well as DIC microscopy for total fungi (Ingham et al. 1989).
Statistical analysis
For all bacterial and fungal data mean, standard error of the mean and parametric paired T-tests were performed using the statistical package SPSS for Windows® Version 6.13 (SPSS Inc. Chicago, IL).
RESULTS
Total bacterial levels did not change in the control from pre to post-test (Figure 1).
Total bacterial levels tended to increase in the sulfur treatment (P = 0.08), yet were significantly higher for the sulfur + oak treatment (P = 0.03) at 21 months post treatment (Figure 1). Fungal levels remained low for the control from pre to post test, however fungal levels increased at 21 months post treatment for both sulfur treatments (sulfur: P = 0.03; sulfur + oak: P = 0.001). Even though both bacterial and fungal levels increased in the two sulfur treatments, not all of the bacteria or fungi present at the site were active. As shown in Table 3, prior to treatment, active bacteria dominated the site and the active fungal to bacterial ratio was low at 0.17. After 21 months of treatment, the ratio of active bacterial biomass to total biomass was low in the two sulfur treatments (sulfur: 0.09 and sulfur +oak: 0.07?g g-1) compared to the control. Fungal activity was reinvigorated and the active fungal to bacterial ratio was higher in the sulfur treatments (Table 3).
article_866_order_4
Fungal hyphal diameter was slightly higher in the sulfur only treatment compared to the control and sulfur plus oak treatments, although further analysis is needed.
The enteric group levels increased only in the sulfur + oak treatment (P = 0.05), but not in the control or sulfur only treatments (Figure 2).
article_866_order_0
article_866_order_1
In contrast, total yeast levels decreased in both sulfur treatments from pre to post-test (sulfur: P = 0.02; sulfur + oak: P = 0.005), but did not change in the control. Coliform and non-coliform levels did not change significantly over time for either of the sulfur treatments or the control.
DISCUSSION AND CONCLUSION
Invasive Asian earthworm species have disrupted nutrient cycles, reduced leaf litter habitat and altered decomposer communities to favor bacteria over fungi in forest ecosystems. Healthy deciduous forest soils are fungal-dominated with a slow steady release of nutrients for seedling growth and survival. We hypothesized that the fungal community would respond most positively to earthworm control treatments which had oak litter amendments to stabilize the soil surface and provide needed habitat. Given the extent of the damage to the site, we questioned whether this combination of amendments would be sufficient to encourage fungal growth and whether the fungal:bacterial ratio would return to healthy forest levels within the time frame of the study. We also predicted that the bacterial activity would decrease given sustained suppression of earthworm activity because earthworms excrete large volumes of bacteria in their casts.
Our data show that total fungal abundance and the ratio of active fungal:bacterial biomass increase when earthworm biomass is suppressed using both sulfur treatments. Microflora play a critical role in the biological activity of most soils in terms of decomposition and humus formation, particle stabilization and nutrient cycling. Fungi tend have larger biomass in many soils ranging from 100 to 1,500 gm-2 compared to bacteria which typically range from 40-500 gm-2 (Brady and Weil 2004). In beech and sugar maple-dominated deciduous forests in Ontario, total fungal abundance was highest in May at 4.3 x 105 CFU g-1 (Keller and Bidochka 1998). Total fungal abundance at our experimental site was more than ten times higher at 1.4 to 2.6 x 106 CFU g-1 prior to treatment and when earthworm populations were suppressed it rose to 28.5 to 32 x 106 CFU g-1 in the two sulfur treatments. Pouyat et al. 1994 found significantly lower litter fungal biomass and hyphal length at urban oak forest sites compared to rural oak forests in a land-use gradient from inner-city New York to rural Connecticut. While Pouyat et al. (1994) found that heavy metal concentrations were higher in these urban forest sites which may impact fungal abundance, a co-current factor was invasive earthworm populations. Densities of predominately Amynthas spp at the urban sites were 25.1 ind m-2 compared to 2.5 ind m-2 at the rural sites (Steinberg et al. 1997). In comparison, at our site we had 95 ind m-2 prior to treatment and 17 ind m-2 in the sulfur treatments 21 months post treatment. Short-term experimental data from other studies concur with our findings. Microbial biomass decreased when earthworms were added to experimental plots in Georgia (Hendrix et al. 1998); whereas microbial biomass increased when earthworm populations were lowered in Ohio (Blair et al. 1997).
While total fungal levels increased in our experiment for both sulfur treatments, yeast levels decreased. Yeasts are common in soil and like other fungi play a role in decomposition (Atlas and Bartha 1998). Levels of yeast in beech and sugar maple-dominated forests in Ontario were 1.5 x 104 CFU g-1 in August (Keller and Bidochka 1998). In temperate deciduous and coniferous forests in Slovakia, forest soils had yeast concentrations of 1.5 x 103 to 1.1 x 104 CFU g-1 (Sláviková and Vadkertiová 2000). In comparison yeast levels were up to ten times lower in tilled agricultural soils in the same region (Sláviková and Vadkertiová 2003). Yeast population levels were much higher in our initial earthworm invaded forest soils (44.4 to 69.6 x 106 CFU g-1) and dropped significantly in the sulfur treatments to 16.8 to 21.4 106 CFU g-1 which is still more than a thousand times higher than in non-earthworm dominated forest soils. Researchers estimate that there are over one million undiscovered fungi species (Brady and Weil 2004). Current studies on yeast in soil are still at the stage of species identification and distribution as opposed to biological function. Little is known about what role yeasts play is nutrient cycling and soil decomposition or how they respond to habitat alterations and disturbance. In our study it is not possible to disaggregate which factor: earthworm biomass suppression, decreased pH or some other factor had the most significant effect on yeast levels. More information is needed on the role of specific yeast species in forest soils and their relationship to invasive earthworm populations.
Healthy deciduous forests tend to have fungal to bacterial biomass ratios greater than 1 and normally near 10 (Ingham et al. 1985). Increases in earthworm populations either through experimental manipulations or via invasion of forest sites have been shown to result in increased bacterial biomass (Wardle 2002; Lavelle et al. 1999; Scheu and Parkinson 1994). In our study, pretreatment active fungal to bacterial biomass ratios were 0.19, but increased to 1.37 (sulfur) and 1.13 (sulfur +oak) after 21 months post treatment. Total bacterial biomass increased, but the ratio of active bacteria decreased. On possible explanation for this trend is that soil microbial populations are sensitive to pH (Gupta 1994). While many fungi species are acid tolerant, most bacteria species are not (Atlas and Bartha 1998). Long-term studies in Finland of acid-rain simulation in a mixed pine-mountain birch woodland indicated that bacterial biomass and activity both decreased at pH levels as low as 3.65, but fungal biomass and activity were not affected (Pennanen et al. 1998). Our treatments were applied in the fall. Soil pH levels dropped below 4.5 at seven months and remained below the 6 to 6.5 range of the control for the entire study. We hypothesize that by 21 months post treatment bacteria died due to either the prolonged low pH, decreased earthworm biomass or the combination of the two factors together. Further research is needed to differentiate the impact of these factors on bacterial activity.
We also measured the levels of human pathogenic microorganisms in the family Enterobacteriaceae which are known to cause such diseases as meningitis, typhoid and food poisoning (Prescott et al. 1999). Earthworms have been used in waste management since the 1970s to render contaminants such as Salmonella enteriditis, fecal coliforms and other human pathogens in the Enterobacteriaceae as innocuous (Edwards and Arancon 2004). Eastman et al. (2001) found that the epigeic earthworm Eisenia fetida reduced enteric levels in sewage sludge by 98% in 72 hours to the acceptable EPA Class A Pathogen Stabilization standards. In our experiment, coliform levels did not change significantly and enteric levels increased only in the sulfur + oak treatment. Although, we did not quantify pathogen levels in relation to EPA standards so can not interpret the human or wildlife health implications. We would have predicted an increase in enterics in both these treatments given that earthworm biomass decreased. Other factors such as low pH may have impacted enteric growth. These species have an optimum growth rate at pH of 6-7 with a minimum tolerance of 4.4 (Doetch and Cook 1973). The pH of the sulfur treatment stayed below 4.5 longer than that of the sulfur + oak treatment.
Our study has documented that management of invasive earthworms is critical for initiating restoration of fungal-dominated temperate forests. When earthworm biomass is suppressed using sulfur amendments, fungal abundance increased and the ratio of active fungal biomass to active bacterial biomass returned to within minimal healthy levels for deciduous forest ecosystems in 21 months. As fungi play such a critical role in tree health and nutrition, earthworm control treatments which result in both suppression of earthworm biomass over time and initiate amelioration of their negative effects on the microbial community speed the forest recovery process. This study is only an initial step in a much longer research process aimed at developing management practices for successful forest restoration.
Our study raises as many questions as it answers. Further research is needed on native plant diversity and seedling growth in relation to altered fungal levels when earthworm biomass is suppressed. How does fungal species composition and metabolic function, particularly yeasts, change during restoration and how critical are these changes to successful plant establishment? As is the case with acid-rain and other disturbed habitat remediation work (Dodd and Thompson 1994; Cordell 1997), would additional fungal inoculation speed the recovery process? Long term studies at Harvard Forest (where fine root distributions and litterfall were manipulated, indicated that significant changes in microbial metabolism took five years to detect (Nadelhoffer et al. 2004). We plan on re-sampling microbial parameters in our treatment plots in three more years to assess whether the initial changes measured in this study continue on the road toward restoration versus re-invasion and subsequent degradation by invasive earthworms.
ACKNOWLEDGEMENTS
Thanks to Richard Tustin III, Luke Bourassa, Evan Halsheid & Jessica Hullit, the previous researchers on the project for lending a very helping hand on the continuation of the research. Staff at the Schuylkill Center for Environmental Education provided background technical reports on the study site and assisted in field data collection.
REFERENCES
Alban D.H. and E.C. Berry (1994) Effects of earthworm invasion on morphology, carbon and nitrogen of a forest soil. Appl. Soil Ecol. 1:243-49.
Atlas R. M. and R. Bartha (1998) Microbial Ecology: Fundamentals and Applications. 4th Edition. Benjamin-Cummings Pub., Menlo Park, CA.
Blair J. M. et al. (1997) Changes in soil N pools in response to earthworm population manipulations in agroecosystems with different N sources. Soil. Biol. Biochem. 29:361-367.
Blair J.M. et al. (1995) Manipulation of earthworm populations in field experiments in agroecosytems. Acta Zool. Fennica 196:48-51.
Bockarie A. et al. (in review) Invasive earthworm management trials for restoration of an urban temperate deciduous forest site. Restoration Ecology.
Bohlen P. et al. (2004) Non-native invasive earthworms as agents of change in northern temperate forests. Front. Ecol. Environ. 2 (8):427-435.
Bouch? M.B. (1977) Strat?gies lombriciennes. In: Lohm U. and T. Persson (eds) Soil organisms as components of ecosystems. Ecol. Bull. (Stockholm) 25:122-132.
Brady N.C. and R. R. Weil (2004) Elements of the Nature and Properties of Soil. 2nd Edition. Pearson Prentice Hall Pub., Upper Saddle River, NJ.
Clements S. and G. Moore (2003) Patterns of species richness in eight northeastern United States cities. Urban Habitats 1(1):4-11.
Coleman D. C. et al. (1992) Soil biology, soil ecology and global change. Biol. Fert. Soils 14:104-111.
Cordell C. E. (1997) Mycorrhizal fungi: beneficial tools for mineland reclamation and Christmas trees. US For Serv Gen Tech Rep PNW. 389:9192.
Dodd J. C. and B. D. Thomson (1994) The screening and selection of inoculant arbuscular-mycorrhizal and ectomycorrhizal fungi. Plant Soil. 159:149158.
Doetch R.N. and T.M. Cook (1973) Introduction to Bacteria and their Ecobiology. University Park Press, Baltimore, MD.
Domínguez J. (2004) State-of-the-art and new perspectives on vermicomposting research. In: Edwards CA (ed) Earthworm Ecology 2nd Edition pp 401-424. CRC Press, Boca Raton, FL.
Eastman B.R. et al. (2001) The effectiveness of vermiculture in human pathogen reduction for USEPA class A stabilization. Compost Sci. Util. 9:38-49.
Edwards C.A. and N.Q. Arancon (2004) The use of earthworms in the breakdown of organic wastes to produce vemicomposts and animal feed protein, pp 345-379. In: Edwards CA (ed) Earthworm Ecology 2nd Edition. CRC Press, Boca Raton, FL.
Edwards C.A. and P.J. Bohlen (1996) Biology and Ecology of Earthworms 3rd Edition, Chapman and Hall, London, UK.
Fisk M.C. et al. (2004) Earthworm invasion, fine root distribution and soil respiration in hardwood forests. Ecosystems 7:55-62.
Gates G.E. (1982) Farewell to North American megadriles. Megadrilogica 4:12-77.
Groffman P.M. et al. (2004) Exotic earthworm invasion and microbial biomass in temperate forest soils. Ecosystems 7:45-54.
Grover A.E. and D. A. Burton (2002) Stilt-grass management in a forest understory. Stilt-grass Management Workshop Schuylkill Center for Environmental Education, Philadelphia, PA.
Gupta V.V.S.R. (1994) The impact of soil and crop management practices on the dynamics of soil microfauna and mesofauna, pp 107-124. In: Pankhurst C.E., Doube B.M., Gupta V.V. S.R. and P.R. Grace (eds.) Soil Biota: Management in Sustainable Farming Systems. CSIRO, East Melbourne, Australia.
Hale C.M. et al. (2005) Exotic European earthworm community composition in northern hardwood forests of Minnesota, USA. Ecol. Appl. 15:848-860.
Hendrix P.F. et al. (1998) Long-term effects of earthworms on microbial biomass nitrogen in course and fine textured soils. Appl. Soil Ecol. 9:375-380.
Hendrix P.F. and P.J. Bohlen (2002) Exotic earthworm invasions in North America: ecological and policy implications. Bioscience 52:801-11.
Ingham E.R. et al. (1989). An analysis of foodweb structure and function in a shortgrass prairie, mountain meadow and lodgepole pine forest. Boil. Fertil. Soils 8:29-37
Ingham R.E. et al. (1985). Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol. Monogr. 55:119-140.
James S.W. and P.F. Hendrix (2004) Invasion of exotic earthworms into North America and other regions., pp. 75-88. In: Edwards, CA. (ed). Earthworm Ecology 2nd Edition. CRC Press, Boca Raton, FL.
Keller L. and M.J. Bidochka (1998) Habitat and temporal differences among soil microfungal assemblages in Ontario. Can. J. Bot. 76(10):1798-1805.
Knollenberg W.G. et al. (1985) Consumption of leaf litter by Lumbricus terrestris (Oligochaeta) on a Michigan woodland floodplain. Am. Mid. Naturalist 113:1-6.
Kozlovskaya L.S. and E.N. Zhdannikova (1961) The combined activity of earthworms and the microflora in forest soils. Dokl. Acad. Nauk SSSR 139:574-576.
Kristufek V. et al. (1993) Actinomycete communities in earthworm guts and surrounding soil. Pedobiologica 37: 379-384.
Lavelle P. et al. (eds.) (1999) Earthworm Management in Tropical Agroecosystems. CABI International, New York, NY.
McLean M.A. and D. Parkinson (1998) Impacts of epigeic earthworm Dendrobaena octaedra on micro-fungal community structure in pine forest floor a mesocosm study. Appl. Soil. Ecol. 8: 61-75.
Nadelhoffer, K.J. et al. (2004) The DIRT experiment: litter and root influences on forest soil organic matter stocks and function. Chapter 15 In: Foster D. and J. Aber (eds.). Synthesis Volume of the Harvard Forest LTER Program. Oxford University Press, Oxford, UK.
NOAA-CIRES (2005) Philadelphia, PA 1961-90 Monthly Means. Climate Diagnostics Center. Boulder, CO. (http://www.cdc.noaa.gov), 3 pp
Pennanen T. et al. (1998) Structure of a microbial community in soil after prolonged addition of low levels of simulated acid rain. Appl Environ Microbiol. 64(6):2173-2180.
Pouyat R.V. et al. (1994) Environmental effects of forest soil-invertebrate and fungal densities in oak stand along an urban-rural land use gradient. Pedobiologia 38: 385-399.
Prescott L.M. et al. (1999) Microbiology 4th Edition. McGraw-Hill Com., New York, NY.
Sauer L.J. (1998) The Once and Future Forest: A Guide to Forest Restoration Strategies Island Press, Washington, DC.
Scheu S. and D. Parkinson (1994) Effects of earthworms on nutrient dynamics, carbon turnover and microorganisms in soils from cool temperate forests of the Canadian Rocky Mountains laboratory studies. Appl. Soil. Ecol. 1:113-125.
Shissler B.P. and G. Seidel (1989) Management Plan for the White-tailed deer at the Schuylkill Center for Environmental Education (SCEE). Consultant Report prepared for the Board of SCEE. Philadelphia, PA, 159 pp.
Steinberg D.A. et al. (1997) Earthworm abundance and nitrogen mineralization rates along an urban-rural land use gradient. Soil Biol. Biochem. 29(3/4):427-430.
Stinson K.A. et al. (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms PLoS Biol 4(5): e140
Sláviková E. and R. Vadkertiová (2003) The diversity of yeasts in the agricultural soil. J. Basic Microbiol. 43(5):430-436.
Sláviková E. and R. Vadkertiová (2000) The occurrence of yeasts in the forest soils. J. Basic Microbiol. 40(3):207-212.
Szlávecz K. et al. (2006) Invasive earthworms and nitrogen cycling in remnant forest patches. Applied Soil. Ecol. 32(1):54-62.
Tompkins E. et al. (1975) Soil Survey of Bucks and Philadelphia Counties, Pennsylvania. USDA Soil Conservation Service. 130 pp
Tortora G.J. et al. (1998) Chapter 6 Microbial Growth, pp. 164-167. In: Microbiology an Introduction, 6th Edition, Benjamin Cummings, New York, NY.
USDA (1999) National Cooperative Soil Survey. Official Series Description. (http://www2.ftw.nrcs.usda.gov/osd/dat/C/CHESTER.html) 6/15/06.
Visser S. (1985) Role of the soil invertebrates in determining the composition of soil microbial communities, pp 297-317. In: Fitter A., Read D. and M.B. Usher (eds) Ecological Interactions in Soil: Plants, Microbes and Animals. Blackwell Scientific Publications, Oxford, UK.
Wardle D. (2002) Communities and Ecosystems: Llinking the Aboveground and Belowground Components. Princeton University Press. Princeton, NJ.
Williamson R.C. and S. Hong (2004) Managing earthworm castings in golf course turf. USGA Turfgrass and Environ. Res. Online. 3(23): 1-6.