Author: Jacob A. Armstrong
Institution: University of Missouri Rolla
Date: February 2005
Abstract
Studies were undertaken to delineate the combustion products of a magnesium (Mg) ribbon on silica (SiO2) crucibles by analyzing the solid surface using X-ray photoelectron spectroscopy (XPS). The sample group included a control, two differently-stained crucibles, and the combustion product, magnesium oxide (MgO). Aluminum (Al) was found in both the raw Mg and the MgO powder, with an oxidation state comparable to that of aluminum oxide (Al2O3). Results of the study revealed evidence leading to the formation of aluminum carbide (Al4C3) as part of the surface integrity.
Introduction
The oxidation reaction of Mg with oxygen (O) on SiO2 surfaces is commonly performed in general chemistry laboratories of many universities, including the University of Missouri - Rolla. When ignited in a Coors ceramic crucible, Mg leaves behind a black carbon (C) like residue, which becomes strongly bonded to the crucible surface as the experiment is repeated. The reaction mechanism for combustion of Mg in air is as follows:
Equation 1
5 Mg(s) + O2(g) + N2(g) + Heat -> 2 MgO(s) + Mg3N2(s)
Equation 2
Mg3N2(s) + 6 H2O(aq) -> 3 Mg(OH)2(s) + 2 NH3(g)
Equation 3
Mg(OH)2(s) + Heat -> MgO(s) + H2O(g)
Despite its appearance in chemistry laboratories across the nation, the precise chemical composition of the residue that remains strongly bonded to the SiO2 crucible surface is unknown. Initial documentation of the solid residue emerged in 1982, and records indicate that the stain is resistant to dissolution via traditional chemical analysis techniques (e.g., atomic absorption, IR, chromatography, mass spectrometry, NMR; Feinstein 1982). As a result, the crucibles used are typically discarded after use. Chemical analysis by means of XPS, a technique for probing solid surfaces, was implemented in order to gain background knowledge of the residue bonds at the surface of the substrate. These experiments are necessary for ultimately devising methods of cleaning the crucible surfaces for repeated use.
Figure 1
XPS is an important method of surface analysis used in numerous fields of study in physics and chemistry, such as microelectronics and heterogeneous catalysis (Chusuei, 2002). This technique probes the energy distribution of electrons ejected from the sample via irradiation caused by X-rays. These electrons provide information about the oxidation state, structure, and composition of the surface, and can be detected on an atomic level in order to provide a surface profile. XPS is also useful for quantitative analysis, as it has capabilities of probing microscopic layers down to one-tenth of a monolayer, or a one-molecule thick quantity of analyte on the surface. The technique is non-destructive; photoelectrons collected and analyzed originate only from the core level of the molecule, leaving the nucleus undisturbed. This feature makes XPS an invaluable tool for elemental composition analysis of solids (Chusuei, 2002).
Figure 2
Materials and Methods
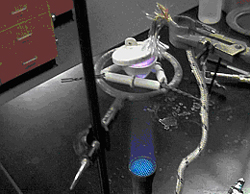
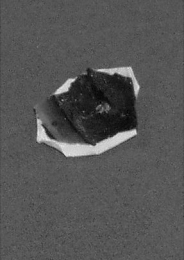
Freshly-stained samples were prepared by igniting ribbons of Mg in ceramic crucibles, according to laboratory procedure (Metz et al. 2004). Four samples were scanned utilizing XPS capabilities: an unused crucible (Control), a hand-prepared crucible in which the Mg ignition was performed once (Crucible 1), a previously used crucible hosting the same ignition numerous times (Pre-Stained), and a portion of the MgO product formed in one of the hand-prepared crucibles (Powder, Crucible 4). The Mg combustion process is shown in Figure 1. Product was extracted from the crucibles after completion of the reaction. Mole ratio in terms of product mass and respective stoichiometry (Equations 1-3) indicated the product to be MgO (APPENDIX A). The crucibles were then broken, and the unidentified black stain remained securely bonded to the crucible surface (Figure 3). In some areas, portions of ceramic had broken away from underneath the deposit, yet bonding integrity between the stain and the top-most layer of the crucible surface remained intact. The broken crucible pieces were then secured to the XPS sample probe in final preparation for analysis (Figure 2).
Figure 3
Results
The XPS survey scans of the four samples, in terms of their binding energies and signal intensity are provided in Figure 4.
Individual element scans of C (1s) and O (1s) are shown in Figure 5 and Figure 6, respectively. Additional detected elements such as Mg (2p), Silicon (Si, 2p), and Al (2p) are noted in the survey scan (Figure 4).
The C detected in each of the XPS scans is known as adventitious carbon, found in airborne hydrocarbons. Silicon carbide (SiC), normally appearing at 275.95 eV, appears on the surface of the Control crucible at ~275.5 eV (Muehlhoff et al., 1986). Industrial ceramics manufacturers use SiC in the glazing process of crucible firing (Boyd, 2003). SiC plays a key role in the formation of Al4C3, seen at ~282 eV in the intensified scan of the Powder (Figure 5a) and having a reference binding energy of 283.4 eV (Hauert, 1993). A slight shift in the oxidation state of C (1s) in the Powder may be attributed to the interactions between Mg, N, and water (H2O). Previously acquired XPS data has shown that H2O readily attacks magnesium nitride (Mg3N2) films, initiating formation of MgO and other hydroxides (Peng et al., 1988). A portion of the C (1s) found initially on the crucible surface reacts with excess O to form a carbonate (CO2) on MgO, which is represented by the peak seen at 290.3 eV in the Powder scan (Onishi et al., 1987).
Figure 4
Oxygen detected during analysis of each crucible is normally attributed to hydroxides, but the powder scan reveals a chemical shift due to formation of a metal oxide. The shift in the oxidation state of O (1s) in the Powder is a direct result of the MgO formation, also sparked by an attack on the Mg3N2 film by H2O (Peng et al., 1988).
The approximate atomic compositions, by percent, for each of the four samples are provided in Table 1. Note that the signal for Crucible 1 was extremely weak and data may not be completely accurate. It was determined that the weak signal (Crucible 1) may be justified by the fact that a single reaction on the crucible surface produces a quantitatively small deposit, and subsequent combustions are required to yield an amount of stain sufficient for XPS analysis.
Table 1
Sample %C(1s) %O(1s) %Mg(2p) %Si(2p) %Al(2p) T(K) t(sec)
Control 29.0 46.7 0 24.3 0 n/a n/a
Crucible 18.20 73.2 17.1 1.50 0 540 600
Pre-Stained 60.0 28.8 5.80 0 5.40 n/a n/a
Powder 22.3 34.0 27.4 0 16.3 855 600
Figure 5
The reaction temperatures were monitored during the experiment by means of a thermocouple, and the average temperature was experimentally determined to be 862 +- 21 K (APPENDIX A). Based on the unusually high combustion temperatures, the silver-grey stain caused by Mg combustion on a crucible surface was determined to be Al4C3. In past experiments, it has been determined that Al4C3 will form catalytically on an aluminum-silicone carbide (AlSiC) interface, when exposed to H2O(g) at 873 K for a period of 600 seconds (Maruyama, 1996). Therefore, an Al4C3 stain is formed on the crucible surface by addition of H2O during the Mg combustion process, which serves to liberate any unreacted Ammonia (NH3) vapors (Equation 2).
Note that the remarkably low temperature recorded for Crucible 1 (500-550 K) can be rationalized by the fact that the thermocouple wires were resting against the side of the crucible and became bonded to the surface during the Mg combustion. As a result, the thermocouple wires were separated at an unknown point during the experiment and the temperature reading was dampened.
The presence of Al in the crucible is attributed to two verifiable sources. A high resolution XPS scan of the Mg ribbon, prior to combustion, revealed impurities of Al and sodium (Na) in the metal (Figure 7).
Although not shown in figures, signals were also detected for Na (1s) and Al (2s). The appearance of orbital pairs provides confirmation of each element, a feature exclusive to XPS. Therefore, the unburned Mg may be identified as the first justified source of Al. It has also been proven that some transition metals, including Al, are an intrinsic impurity of SiC interfaces (Lambrecht, 2000). Therefore, the SiC crucible coating provides a second supportable source of Al. The concentration of Al in SiC has been quantitatively determined to be about 9.1 parts per billion by mass, via Gas Discharge Mass Spectrometry (CVD Silicon Carbide®).
In addition to these two documented Al sources, it can be speculated that the crucibles themselves may contribute to the Al impurity, as crucibles are commonly composed of Al2O3.
Discussion
The Control crucible was not physically altered before being analyzed by XPS. Elements detected included C (1s), O (1s), and Si (2p).
Figure 6
For Crucible 1, a single combustion of Mg was performed on the ceramic surface. Elements detected in XPS were: C (1s), O (1s), Mg (2p), and trace amounts of Si (2p). However, the C (1s) and Al (2p) signals were severely attenuated by the top-most surface formation of MgO.
The Pre-Stained crucible hosted an unknown number of Mg combustion reactions on a common ceramic surface. Large quantities of C (1s) were detected, with attenuating O (1s) and significant Mg (2p) content. The atomic ratio of Al (2p) to Mg (2p) on the Pre-Stained surface, as determined by XPS, was approximately 1:1.
Figure 7
The MgO Powder extracted from Crucible 4 represents the products of Mg combustion in air. Analysis of this product using XPS resulted in the detection of C (1s), O (1s), Mg (2p), and surprisingly, Al (2p). The atomic ratio of Mg (2p) to O (1s) in the product was approximately 1:1, complementing the mole ratio of the MgO product (Metz et al., 2004). Again, the presence of Al (2p) in the MgO product is attributed to impurities within the Mg itself, along with impurities found in SiC.
In summary, the black stain present on a crucible surface as a result of Mg ignition in the presence of air is postulated to be Al4C3, also known as aluminum carbide. Development of a successful method for removal of this stain from a crucible surface is left as a topic for further research. When considering its removal, the structures of Al4C3 should be researched and considered.
Acknowledgements
I would like to thank my research advisor, Dr. Charles C. Chusuei, for his assistance involving XPS analysis of the samples and editing of the final report. I also thank Dr. Terry Bone for providing crucibles (new and used), Mg strips, and equipment necessary for the Mg combustion experiment, Dr. Phil Whitefield for providing additional direction in the research of carbide formations, and Mr. Ahmed Shahin for obtaining photographs of various crucible samples.
References
Feinstein HI. (1982). "Ignition of Magnesium in Porcelain." Journal of Chemical Education. 59:159.
Chusuei CC. (2002). "X-Ray Photoelectron Spectroscopy." Encyclopedia of Physical Science and Technology. 17:921-938.
Muehlhoff L. et al. (1986). "Comparative Electron Spectroscopic Studies of Surface Segregation on SiC(0001) and SiC(000 )" Journal of Applied Physics. 60;8:2842-2853.
Boyd M. (2003). Coors Ceramics Company Email Inquiry. 14 Nov. 2003 http://www.processregister.com/Coors%20Ceramics%20Company/supplier/sid305.
Waldrop JR., R.W Grant,. (1990). "Formation and Schottky Barrier Height of Metal Contacts to -SiC." Applied Physics Letters. 56:557-559
Peng XD, DS Edwards, MA Barteau.. (1988). "Central Catalog of Science and Technology." Surface Science. 195:1-2;103:14.
Onishi H. et al. (1987). "Adsorption of Na Atoms and Oxygen Containing Molecules on MgO(100) and (111) Surfaces." Surface Science. 191;3:479-491.
Maruyama B. (1996). "H2O Catalysis of Aluminum Carbide Formation in the Aluminum-Silicon Carbide System." Journal of Materials Research. 6.6:1131.
Lambrecht WR. (2000). First-Principles Theory of Transition Metal Impurities in Silicon Carbide. Case Western: University of Cleveland.
CVD Silicon Carbide®. 6 June, 2004 http://www.cvdmaterials.com/sicprop1.htm
Metz CR. et al. (2004). Chemistry Lab Experiments. Pacific Grove: Brooks/Cole.
Wagner CD. et al. (1979). Handbook of X-Ray Photoelectron Spectroscopy. Eden Prairie: Perkin-Elmer Corporation.
Appendix A
The atomic ratio of Magnesium to Oxygen in each molecule for crucibles 1-5, respectively, was determined from the experimental data as shown in Figure 8 and Figure 9.
Figure 8
Figure 9
The reaction temperature of Magnesium ignition in a ceramic crucible was determined from the experimental data as shown in figure , using the "Q-test" to eliminate outliers.